Organic radicals with inversion of SOMO and HOMO energies and potential applications in optoelectronics
- PMID: 36128246
- PMCID: PMC9430691
- DOI: 10.1039/d2sc02480b
Organic radicals with inversion of SOMO and HOMO energies and potential applications in optoelectronics
Abstract
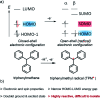
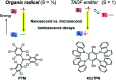
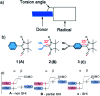
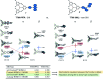
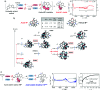
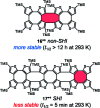
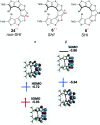
Organic radicals possessing an electronic configuration in which the energy of the singly occupied molecular orbital (SOMO) is below the highest doubly occupied molecular orbital (HOMO) level have recently attracted significant interest, both theoretically and experimentally. The peculiar orbital energetics of these SOMO-HOMO inversion (SHI) organic radicals set their electronic properties apart from the more common situation where the SOMO is the highest occupied orbital of the system. This review gives a general perspective on SHI, with key fundamental aspects regarding the electronic and structural factors that govern this particular electronic configuration in organic radicals. Selected examples of reported compounds with SHI are highlighted to establish molecular guidelines for designing this type of radical, and to showcase the potential of SHI radicals in organic spintronics as well as for the development of more stable luminescent radicals for OLED applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


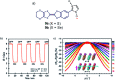
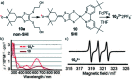

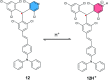

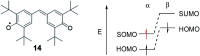


References
-
- Williams B. B. and Halpern H. J., Biomedical EPR – Part A: Free Radicals, Metals, Medicine and Physiology, Springer US, 2005
-
- Chen Z. X. Li Y. Huang F. Chem. 2021;7:288–332.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

