Synthesis and evaluation of the antioxidant activity of 3-pyrroline-2-ones: experimental and theoretical insights
- PMID: 36128396
- PMCID: PMC9425838
- DOI: 10.1039/d2ra04640g
Synthesis and evaluation of the antioxidant activity of 3-pyrroline-2-ones: experimental and theoretical insights
Abstract
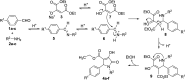
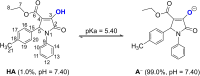
The heterocyclic γ-lactam ring 2-pyrrolidinone has four carbon atoms and one nitrogen atom. Among the group of derivatives of 2-pyrrolidinones, 1,5-dihydro-2H-pyrrol-2-ones, also known as 3-pyrroline-2-ones, play a significant structural role in a variety of bioactive natural compounds. In this study, three-component reactions were used to successfully synthesize six polysubstituted 3-hydroxy-3-pyrroline-2-one derivatives. The antioxidant activity of the compounds was tested by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, identifying 4-ethoxycarbonyl-3-hydroxy-5-(4-methylphenyl)-1-phenyl-3-pyrroline-2-one (4b) as the most promising radical scavenger. Quantum chemistry calculations of the thermodynamics and kinetics of the radical scavenging activity also suggest that 4b is an effective HO˙ radical scavenger, with k overall values of 2.05 × 109 and 1.54 × 1010 M-1 s-1 in pentyl ethanoate and water, respectively. On the other hand, 4b could not scavenge hydroperoxyl radicals in either media. The ability of 4b to scavenge hydroxyl radicals in polar and non-polar environments is comparable to that of conventional antioxidants such as melatonin, gallic acid, indole-3-carbinol, ramalin, or Trolox. Thus 4b may be classed as a promising HO˙ radical scavenger in the physiological environment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Singh S. B. Zink D. L. Goetz M. A. Dombrowski A. W. Polishook J. D. Hazuda D. J. Tetrahedron Lett. 1998;39:2243–2246. doi: 10.1016/S0040-4039(98)00269-X. - DOI
LinkOut - more resources
Full Text Sources

