Cytospin performance when using Paris system for reporting urinary cytology
- PMID: 36128466
- PMCID: PMC9479516
- DOI: 10.25259/Cytojournal_48_2021
Cytospin performance when using Paris system for reporting urinary cytology
Abstract
Objectives: The Paris System (TPS) for Reporting Urine Cytology has significantly improved the approach to evaluating urine cytology. TPS criteria were defined mainly according to ThinPrep and SurePath preparations, as they are widely utilized. The objective of this study is to validate urine cytology interpretation according to the TPS classification using cytospin technique in relation to the gold slandered histology.
Material and methods: This retrospective study examined and analyzed 316 urine specimens from King Abdulaziz University Hospital between 2015 and 2020. Cytospin technique is performed for all cases. Slides were recategorized using TPS criteria, then compared with the original histology diagnosis.
Results: According to the TPS, 108 cases were classified as 101 AUC (32%), 95 NEG (30%), 59 HGUC (18.7%), 31 SHGUC (9.8%), and 30 (9.5%) others. The computed sensitivity of cytospin in urine cytology was 94.7%, with 73.9% specificity, a positive predictive value of 85.6%, a negative predictive value of 89.5%, and overall accuracy of 86.8%.
Conclusion: Urine cytology testing is considered to be a non-invasive and sensitive method to screen for urothelial carcinoma. TPS defined standards are reliable on cytospin prepared slides for reporting urine cytology.
Keywords: Conventional cytospin; PARIS system; Urine cytology.
© 2022 Cytopathology Foundation Inc, Published by Scientific Scholar.
Conflict of interest statement
No potential competing interest was reported by all authors.
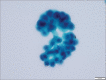
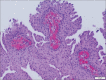
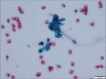
Figures




Similar articles
-
Comparison of conventional and liquid-based cytology using The Paris System for Reporting Urinary Cytology.Cytopathology. 2021 Nov;32(6):795-801. doi: 10.1111/cyt.13040. Epub 2021 Aug 4. Cytopathology. 2021. PMID: 34289188
-
Split-sample comparison of urothelial cells in ThinPrep and cytospin preparations in urinary cytology: Do we need to adjust The Paris System for Reporting Urinary Cytology criteria?Cancer Cytopathol. 2020 Feb;128(2):119-125. doi: 10.1002/cncy.22218. Epub 2019 Nov 27. Cancer Cytopathol. 2020. PMID: 31774630
-
Utility of the Paris System in Urine Cytology for Improved Screening of High-Grade Urothelial Carcinoma in Bahrain.Cureus. 2024 Mar 29;16(3):e57189. doi: 10.7759/cureus.57189. eCollection 2024 Mar. Cureus. 2024. PMID: 38681345 Free PMC article.
-
Experience on the use of The Paris System for Reporting Urinary Cytopathology: review of the published literature.J Am Soc Cytopathol. 2021 Jan-Feb;10(1):79-87. doi: 10.1016/j.jasc.2020.10.002. Epub 2020 Oct 10. J Am Soc Cytopathol. 2021. PMID: 33160893
-
The Paris System for Reporting Urinary Cytology: A Meta-Analysis.J Pers Med. 2022 Jan 27;12(2):170. doi: 10.3390/jpm12020170. J Pers Med. 2022. PMID: 35207658 Free PMC article. Review.
References
-
- Layfield LJ, Elsheikh TM, Fili A, Nayar R, Shidham V, Papanicolaou Society of Cytopathology Review of the state of the art and recommendations of the papanicolaou society of cytopathology for urinary cytology procedures and reporting: The papanicolaou society of cytopathology practice guidelines task force. Diagn Cytopathol. 2004;30:24–30. doi: 10.1002/dc.10401. - DOI - PubMed
-
- Khan R, Hussain H, Pambuccian S, Wojcik E, Barkan G. What is the negative predictive value of urinary tract cytology. J Am Soc Cytopathol. 2015;6:S21–2. doi: 10.1016/j.jasc.2015.09.049. - DOI
-
- Rosenthal DL, Wojcik EM, Kurtycz DF. Cham, Switzerland: Springer International Publishing; 2016. The Paris System for Reporting Urinary Cytology. - DOI
LinkOut - more resources
Full Text Sources
