Effects of coordinating heteroatoms on molecular structure, thermodynamic stability and redox behavior of uranyl(vi) complexes with pentadentate Schiff-base ligands
- PMID: 36128519
- PMCID: PMC9413499
- DOI: 10.1039/d2ra04639c
Effects of coordinating heteroatoms on molecular structure, thermodynamic stability and redox behavior of uranyl(vi) complexes with pentadentate Schiff-base ligands
Abstract
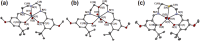
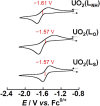
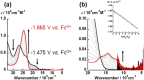
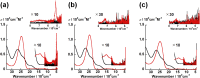
Uranyl(vi) complexes with pentadentate N3O2-, N2O3- and N2O2S1-donating Schiff base ligands, tBu,MeO-saldien-X2- (X = NH, O and S), were synthesized and thoroughly characterized by 1H NMR, IR, elemental analysis, and single crystal X-ray diffraction. The crystal structures of UO2(tBu,MeO-saldien-X) showed that the U-X bond strength follows U-O ≈ U-NH > U-S. Conditional stability constants (β X) of UO2(tBu,MeO-saldien-X) in ethanol were investigated to understand the effect of X on thermodynamic stability. The log β X decrease in the order of UO2(tBu,MeO-saldien-NH) (log β NH = 10) > UO2(tBu,MeO-saldien-O) (log β O = 7.24) > UO2(tBu,MeO-saldien-S) (log β S = 5.2). This trend cannot be explained only by Pearson's Hard and Soft Acids and Bases (HSAB) principle, but rather follows the order of basicity of X. Theoretical calculations of UO2(tBu,MeO-saldien-X) suggested that the ionic character of U-X bonds decreases in the order of U-NH > U-O > U-S, while the covalency increases in the order U-O < U-NH < U-S. Redox potentials of all UO2(tBu,MeO-saldien-X) in DMSO were similar to each other regardless of the difference in X. Spectroelectrochemical measurements and DFT calculations revealed that the center U6+ of each UO2(tBu,MeO-saldien-X) undergoes one-electron reduction to afford the corresponding uranyl(v) complex. Consequently, the difference in X of UO2(tBu,MeO-saldien-X) affects the coordination of tBu,MeO-saldien-X2- with UO2 2+. However, the HSAB principle is not always prominent, but the Lewis basicity and balance between ionic and covalent characters of the U-X interactions are more relevant to determine the bond strengths.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Casellato U. Vidali M. Vigato P. A. Coord. Chem. Rev. 1979;28:231–277. doi: 10.1016/S0010-8545(00)82015-9. - DOI
-
- Ephritikhine M. Coord. Chem. Rev. 2016;319:35–62. doi: 10.1016/j.ccr.2016.04.020. - DOI
-
- Thamer B. J. J. Am. Chem. Soc. 1957;79:4298–4305. doi: 10.1021/ja01573a016. - DOI
-
- Bullita E. Guerriero P. Tamburini S. Vigato P. A. Dupuy J. C. Prevost M. Bonora R. Marchesini L. Mater. Chem. Phys. 1992;31:181–198. doi: 10.1016/0254-0584(92)90175-8. - DOI
LinkOut - more resources
Full Text Sources

