Fe(iii)-catalysed selective C-N bond cleavage of N-phenylamides by an electrochemical method
- PMID: 36128521
- PMCID: PMC9403817
- DOI: 10.1039/d2ra04709h
Fe(iii)-catalysed selective C-N bond cleavage of N-phenylamides by an electrochemical method
Abstract
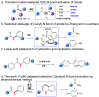
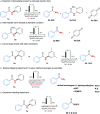
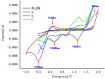
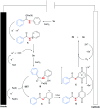
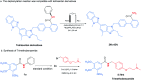
An Fe(iii)-catalysed transformation of secondary N-phenyl substituted amides to primary amides by an electrochemical method is developed. Regioselective aryl C-H oxygenation occurs during the reaction, promoting selective C(phenyl)-N bond cleavage to form primary amides in yields of up to 92%.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
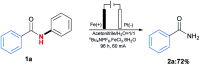
Similar articles
-
Nickel-catalysed direct alkylation of thiophenes via double C(sp3)-H/C(sp2)-H bond cleavage: the importance of KH2PO4.Chem Commun (Camb). 2017 Jul 20;53(59):8316-8319. doi: 10.1039/c7cc04252c. Chem Commun (Camb). 2017. PMID: 28686241
-
Product Control using Substrate Design: Ruthenium-Catalysed Oxidative C-H Olefinations of Cyclic Weinreb Amides.Chemistry. 2016 Nov 14;22(47):16986-16990. doi: 10.1002/chem.201603126. Epub 2016 Oct 10. Chemistry. 2016. PMID: 27723223
-
A new tripodal iron(III) monophenolate complex: effects of ligand basicity, steric hindrance, and solvent on regioselective extradiol cleavage.Inorg Chem. 2007 Jul 23;46(15):6038-49. doi: 10.1021/ic700646m. Epub 2007 Jun 23. Inorg Chem. 2007. PMID: 17589990
-
Selective C(sp2)-C(sp) bond cleavage: the nitrogenation of alkynes to amides.Angew Chem Int Ed Engl. 2013 Jul 22;52(30):7850-4. doi: 10.1002/anie.201303376. Epub 2013 Jun 26. Angew Chem Int Ed Engl. 2013. PMID: 23804537
-
Selective Cleavage of Inert Aryl C-N Bonds in N-Aryl Amides.J Org Chem. 2018 Feb 2;83(3):1369-1376. doi: 10.1021/acs.joc.7b02880. Epub 2018 Jan 17. J Org Chem. 2018. PMID: 29302966
References
-
- Li G. Szostak M. Chem. Rec. 2020;20:649. - PubMed
- Li G. Szostak M. Nat. Commun. 2018;9:4165. - PMC - PubMed
- Gao P. Szostak M. Org. Lett. 2020;22:6010. - PubMed
- Rahman M. M. Szostak M. Org. Lett. 2021;23:4818. - PubMed
- Meng G. Szostak M. Org. Lett. 2016;18:796. - PubMed
- Lei P. Meng G. Shi S. Ling Y. An J. Szostak R. Szostak M. Chem. Sci. 2017;8:6525. - PMC - PubMed
- Zhou T. Li G. Nolan S. P. Szostak M. Org. Lett. 2019;21:3304. - PubMed
-
- Sanderson R. T., Polar Covalence; Academic Press: New York, 1983
- Sanderson R. T., Chemical Bonds and Bond Energy; Academic Press: New York, 1976
LinkOut - more resources
Full Text Sources

