Insight into nanocrystal synthesis: from precursor decomposition to combustion
- PMID: 36128523
- PMCID: PMC9425161
- DOI: 10.1039/d2ra05222a
Insight into nanocrystal synthesis: from precursor decomposition to combustion
Abstract
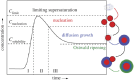
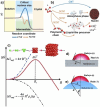
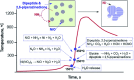
Nanotechnology-based synthesis of nanoscale materials has appealed to the attention of scientists in the modern scientific community. In the bottom-up approach, atoms start to aggregate/agglomerate and form nuclei within the minimum and maximum supersaturation range. Once nuclei are generated above the critical-free energy/radius, the growth is initiated by obeying the LaMar model with a slight extra simple growth by diffusion advancement. The in situ real-time liquid phase analysis using STEM, AFM, and XAS techniques is used to control precursor decomposition to the nanocrystal formation process and should be a non-stoppable technique. Solution combustion synthesis (SCS) is a time-/energy-efficient self-sustained process that produces mass-/ion transport active porous materials. SCS also permits the synthesis of evenly distributed-doped and hybrid-nanomaterials, which are beneficial in tuning crucial properties of the materials. The growth and development of nanocrystals, dehydrating the sol in the presence of a surfactant or/and fuel results in combustion once it arrives at the ignition temperature. Besides, the kinetic and thermodynamics controlled architecture-directing agent-assisted SCS offers colloidal nanocrystal framework formation, which is currently highly applicable for energy devices. This short review provides insightful information that adds to the existing nanocrystal synthesis process and solution combustion synthesis and recommends future directions in the field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures















References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

