Synthesis of benzothiazole-appended bis-triazole-based structural isomers with promising antifungal activity against Rhizoctonia solani
- PMID: 36128524
- PMCID: PMC9425831
- DOI: 10.1039/d2ra04465j
Synthesis of benzothiazole-appended bis-triazole-based structural isomers with promising antifungal activity against Rhizoctonia solani
Abstract
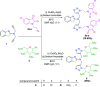
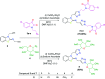
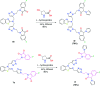
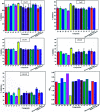
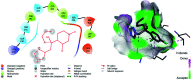
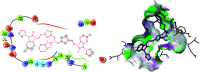
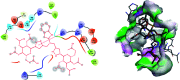
In order to explore new antifungal agrochemicals, we reported the synthesis of two series 5a-f, 6 and 7a-f, 8 of benzothiazole-appended bis-triazole derivative-based structural isomers using a molecular hybridization approach. The synthesized compounds were tested for fungal growth inhibition against the plant pathogen Rhizoctonia solani. All the synthesized compounds showed excellent antifungal activity in their minimum concentrations (10-0.62 μM). Among all the synthetics, compounds 5b (ED50: 2.33 μM), 5f (ED50: 0.96 μM), and 7f (ED50: 1.48 μM) exerted a superior inhibitory effect in comparison to the commercially available fungicide, hexaconazole (ED50: 2.44 μM). The binding interactions of the active compounds 5f, 7f, 6, and 8 within the active site of the sterol 14α-demethylase enzyme were studied with the help of molecular docking studies. The studies revealed that these hybrid pharmacophores could be used as an important intermediate to demonstrate new structural isomer-based fungicides.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures
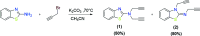



Similar articles
-
New 1,2,3-Triazole-Appended Bis-pyrazoles: Synthesis, Bioevaluation, and Molecular Docking.ACS Omega. 2021 Sep 13;6(38):24879-24890. doi: 10.1021/acsomega.1c03734. eCollection 2021 Sep 28. ACS Omega. 2021. PMID: 34604669 Free PMC article.
-
Design, Synthesis, and Antifungal Activities of Novel Pyrazole-4-carboxamide Derivatives Containing an Ether Group as Potential Succinate Dehydrogenase Inhibitors.J Agric Food Chem. 2023 Jun 21;71(24):9255-9265. doi: 10.1021/acs.jafc.3c00116. Epub 2023 Jun 7. J Agric Food Chem. 2023. PMID: 37283465
-
Green design and synthesis of some novel thiazolidinone appended benzothiazole-triazole hybrids as antimicrobial agents.RSC Adv. 2024 Mar 12;14(12):8341-8352. doi: 10.1039/d4ra00990h. eCollection 2024 Mar 6. RSC Adv. 2024. PMID: 38476177 Free PMC article.
-
Synthesis and antifungal activity of 2-chloromethyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro.J Agric Food Chem. 2013 Mar 20;61(11):2789-95. doi: 10.1021/jf3053934. Epub 2013 Mar 6. J Agric Food Chem. 2013. PMID: 23419161
-
Fungal Lanosterol 14α-demethylase: A target for next-generation antifungal design.Biochim Biophys Acta Proteins Proteom. 2020 Mar;1868(3):140206. doi: 10.1016/j.bbapap.2019.02.008. Epub 2019 Mar 6. Biochim Biophys Acta Proteins Proteom. 2020. PMID: 30851431 Review.
Cited by
-
Research Progress of Benzothiazole and Benzoxazole Derivatives in the Discovery of Agricultural Chemicals.Int J Mol Sci. 2023 Jun 28;24(13):10807. doi: 10.3390/ijms241310807. Int J Mol Sci. 2023. PMID: 37445983 Free PMC article.
-
Design, synthesis, molecular docking and DFT studies on novel melatonin and isatin based azole derivatives.RSC Adv. 2023 Sep 14;13(39):27525-27534. doi: 10.1039/d3ra05531k. eCollection 2023 Sep 8. RSC Adv. 2023. PMID: 37720826 Free PMC article.
-
Emerging Trends in Neuroblastoma Diagnosis, Therapeutics, and Research.Mol Neurobiol. 2025 May;62(5):6423-6466. doi: 10.1007/s12035-024-04680-w. Epub 2025 Jan 13. Mol Neurobiol. 2025. PMID: 39804528 Review.
-
Oxalactam A, a Novel Macrolactam with Potent Anti-Rhizoctonia solani Activity from the Endophytic Fungus Penicillium oxalicum.Molecules. 2022 Dec 12;27(24):8811. doi: 10.3390/molecules27248811. Molecules. 2022. PMID: 36557941 Free PMC article.
References
-
- Ristaino J. B. Anderson P. K. Bebber D. P. Brauman K. A. Cunniffe N. J. Fedoroff N. V. Finegold C. Garrett K. A. Gilligan C. A. Jones C. M. Martin M. D. MacDonald G. K. Neenan P. Records A. Schmale D. G. Tateosian L. Wei Q. Proc. Natl. Acad. Sci. U. S. A. 2021;118:2–9. doi: 10.1073/pnas.2022239118. - DOI - PMC - PubMed
-
- Iqbal Z. Khan M. A. Sharif M. Shah J. H. ur Rehman M. H. Javed K. Comput. Electron. Agric. 2018;153:12–32. doi: 10.1016/j.compag.2018.07.032. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

