Randomized Trial of Tocilizumab in the Treatment of Refractory Adult Polymyositis and Dermatomyositis
- PMID: 36128663
- PMCID: PMC9661830
- DOI: 10.1002/acr2.11493
Randomized Trial of Tocilizumab in the Treatment of Refractory Adult Polymyositis and Dermatomyositis
Abstract
Objective: To assess the efficacy and tolerability of tocilizumab in a multicenter, randomized, double-blind, placebo-controlled trial in refractory adult patients with dermatomyositis (DM) and polymyositis (PM).
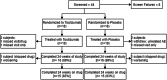
Methods: Thirty-six subjects with probable or definite DM/PM were enrolled in a 6-month phase 2B clinical trial and randomized 1:1 to receive tocilizumab (8 mg/kg intravenously) or placebo every 4 weeks for 24 weeks. Eligible subjects had either a DM rash, a myositis-associated autoantibody or an adjudicated PM diagnosis. Active disease was defined by at least three of six abnormal core set measures (CSMs), including a manual muscle testing (MMT)-8 score of less than 136/150. If the MMT-8 score was greater than 136, then a cutaneous score of 3 or more (10 cm visual analogue scale) was required along with three additional abnormal CSMs indicating disease activity. The primary endpoint compared the Total Improvement Score (TIS) between both arms from week 4 to 24. Secondary outcomes included time to meeting minimal TIS improvement, changes in CSMs, time to worsening, steroid-sparing effect, proportion of subjects meeting more stringent improvement criteria, and safety outcomes.
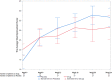
Results: There was no significant difference (P = 0.86) in the TIS over 24 weeks between tocilizumab and placebo arms. The secondary endpoints of time to improvement (minimal, moderate, or major), time to worsening, CSM changes, safety outcomes, and steroid-sparing effect were also not significantly different between arms.
Conclusion: Tocilizumab was safe and well tolerated but did not meet the primary or secondary efficacy outcomes in refractory DM and PM in this 24-week phase 2B study.
© 2022 The Authors. ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Aggarwal R, Rider LG, Ruperto N, Bayat N, Erman B, Feldman BM, et al. 2016 American College of Rheumatology/European League against rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: an international myositis assessment and clinical studies group/paediatric rheumatology international trials organisation collaborative initiative. Ann Rheum Dis 2017;69:898–910. - PMC - PubMed
-
- Kaly L, Rosner I. Tocilizumab ‐ a novel therapy for non‐organ‐specific autoimmune diseases. Best Pract Res Clin Rheumatol 2012;26:157–65. - PubMed
-
- Schoels MM, van der Heijde D, Breedveld FC, Burmester GR, Dougados M, Emery P, et al. Blocking the effects of interleukin‐6 in rheumatoid arthritis and other inflammatory rheumatic diseases: systematic literature review and meta‐analysis informing a consensus statement. Ann Rheum Dis 2013;72:583–9. - PMC - PubMed
-
- Smolen JS, Schoels MM, Nishimoto N, Breedveld FC, Burmester GR, Dougados M, et al. Consensus statement on blocking the effects of interleukin‐6 and in particular by interleukin‐6 receptor inhibition in rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis 2012;72:482–92. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

