"Clickable" Polymer Brush Interfaces: Tailoring Monovalent to Multivalent Ligand Display for Protein Immobilization and Sensing
- PMID: 36128725
- PMCID: PMC9501913
- DOI: 10.1021/acs.bioconjchem.2c00298
"Clickable" Polymer Brush Interfaces: Tailoring Monovalent to Multivalent Ligand Display for Protein Immobilization and Sensing
Abstract
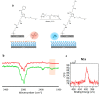
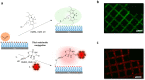
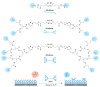
Facile and effective functionalization of the interface of polymer-coated surfaces allows one to dictate the interaction of the underlying material with the chemical and biological analytes in its environment. Herein, we outline a modular approach that would enable installing a variety of "clickable" handles onto the surface of polymer brushes, enabling facile conjugation of various ligands to obtain functional interfaces. To this end, hydrophilic anti-biofouling poly(ethylene glycol)-based polymer brushes are fabricated on glass-like silicon oxide surfaces using reversible addition-fragmentation chain transfer (RAFT) polymerization. The dithioester group at the chain-end of the polymer brushes enabled the installation of azide, maleimide, and terminal alkene functional groups, using a post-polymerization radical exchange reaction with appropriately functionalized azo-containing molecules. Thus, modified polymer brushes underwent facile conjugation of alkyne or thiol-containing dyes and ligands using alkyne-azide cycloaddition, Michael addition, and radical thiol-ene conjugation, respectively. Moreover, we demonstrate that the radical exchange approach also enables the installation of multivalent motifs using dendritic azo-containing molecules. Terminal alkene groups containing dendrons amenable to functionalization with thiol-containing molecules using the radical thiol-ene reaction were installed at the interface and subsequently functionalized with mannose ligands to enable sensing of the Concanavalin A lectin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Wei Q.; Haag R. Universal Polymer Coatings and Their Representative Biomedical Applications. Mater. Horiz. 2015, 2, 567–577. 10.1039/C5MH00089K. - DOI
-
- Seemann A.; Akbaba S.; Buchholz J.; Turkkan S.; Tezcaner A.; Woche S. K.; Guggenberger G.; Kirschning A.; Drager G. RGD-Modified Titanium as an Improved Osteoinductive Biomaterial for Use in Dental and Orthopedic Implants. Bioconjugate Chem. 2022, 33, 294–300. 10.1021/acs.bioconjchem.1c00509. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

