Isolation and analysis of tumor‑derived extracellular vesicles from head and neck squamous cell carcinoma plasma by galectin‑based glycan recognition particles
- PMID: 36129151
- PMCID: PMC9507089
- DOI: 10.3892/ijo.2022.5423
Isolation and analysis of tumor‑derived extracellular vesicles from head and neck squamous cell carcinoma plasma by galectin‑based glycan recognition particles
Abstract
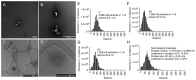
Extracellular vesicles (EVs) have recently come into the spotlight as potential cancer biomarkers. Isolation of pure EVs is complex, so wider use requires reliable and time‑efficient isolation methods. In the present study, galectin‑based magnetic glycan recognition particles, EXÖBead® were investigated for their practicality as a novel EV isolation technique, exemplified here for squamous cell carcinoma of the head and neck. Analysis of the isolation method showed a high concentration of pure EVs with detection of specific EV markers such as CD9, CD63, CD81 and TSG101. No apolipoprotein A1 was shown in the isolates, indicating low contamination of this isolation technique compared with size exclusion chromatography. In addition, common leukocyte antigen (CD45), three HNSCC [epithelial cell adhesion molecule (EpCAM), pan‑cytokeratin and programmed death‑ligand 1 (PD‑L1)] and PanEV markers (premixed CD9, CD63 and CD81 antibodies) were measured by bead‑based flow cytometry (BFC). BFC revealed that CD45Neg PanEV+, EpCAM+ PanEV+ and PD‑L1+ PanEV+ were significantly higher in tumor patients compared with healthy control plasma. CD45Neg PanEV+ and CD45+ PanEV+ carrying two or three HNSCC biomarkers were also significantly higher in tumor patients compared with healthy controls (BFC). Comparison of the functional immunosuppression effect of eluted tumor patient plasma EVs from EXÖBead® and commercial polyethylene glycol isolation showed a significant tumor‑dependent increase in concentration of EVs. A peripheral blood mononuclear cell activation assay also showed that the T‑cell functionality of tumor patient plasma EVs isolated with EXÖBead® was preserved in vitro. In conclusion, isolation using galectin‑based magnetic glycan recognition particles is a novel method for isolating plasma EVs with low lipoprotein contamination. Bead‑based flow cytometry provided an easy way to understand EV subpopulations. EXÖBead® therefore showed great potential as a new isolation tool with high throughput capacity that could potentially be used in a clinical setting.
Keywords: beads‑based flow cytometry; biomarker; exosomes; extracellular vesicles; galectin‑based glycan recognition particles; head and neck squamous cell carcinoma; novel isolation technique; tumor‑derived extracellular vesicles.
Conflict of interest statement
DC is the founder of Biovesicle, Inc. All experiments were conducted without financial contribution, simply scientific advices were obtained. LM and MWP are scientific advisor of Biovesicle, Inc. and did not receive profit.
Figures





References
-
- Lopatina T, Favaro E, Danilova L, Fertig EJ, Favorov AV, Kagohara LT, Martone T, Bussolati B, Romagnoli R, Albera R, et al. Extracellular vesicles released by tumor endothelial cells spread immunosuppressive and transforming signals through various recipient cells. Front Cell Dev Biol. 2020;8:698. doi: 10.3389/fcell.2020.00698. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
