Functional and Structural Characterization of OXA-935, a Novel OXA-10-Family β-Lactamase from Pseudomonas aeruginosa
- PMID: 36129295
- PMCID: PMC9578422
- DOI: 10.1128/aac.00985-22
Functional and Structural Characterization of OXA-935, a Novel OXA-10-Family β-Lactamase from Pseudomonas aeruginosa
Abstract
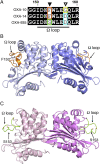
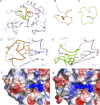
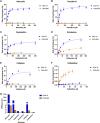
Resistance to antipseudomonal penicillins and cephalosporins is often driven by the overproduction of the intrinsic β-lactamase AmpC. However, OXA-10-family β-lactamases are a rich source of resistance in Pseudomonas aeruginosa. OXA β-lactamases have a propensity for mutation that leads to extended spectrum cephalosporinase and carbapenemase activity. In this study, we identified isolates from a subclade of the multidrug-resistant (MDR) high risk P. aeruginosa clonal complex CC446 with a resistance to ceftazidime. A genomic analysis revealed that these isolates harbored a plasmid containing a novel allele of blaOXA-10, named blaOXA-935, which was predicted to produce an OXA-10 variant with two amino acid substitutions: an aspartic acid instead of a glycine at position 157 and a serine instead of a phenylalanine at position 153. The G157D mutation, present in OXA-14, is associated with the resistance of P. aeruginosa to ceftazidime. Compared to OXA-14, OXA-935 showed increased catalytic efficiency for ceftazidime. The deletion of blaOXA-935 restored the sensitivity to ceftazidime, and susceptibility profiling of P. aeruginosa laboratory strains expressing blaOXA-935 revealed that OXA-935 conferred ceftazidime resistance. To better understand the impacts of the variant amino acids, we determined the crystal structures of OXA-14 and OXA-935. Compared to OXA-14, the F153S mutation in OXA-935 conferred increased flexibility in the omega (Ω) loop. Amino acid changes that confer extended spectrum cephalosporinase activity to OXA-10-family β-lactamases are concerning, given the rising reliance on novel β-lactam/β-lactamase inhibitor combinations, such as ceftolozane-tazobactam and ceftazidime-avibactam, to treat MDR P. aeruginosa infections.
Keywords: OXA-β-lactamase; Pseudomonas aeruginosa; antimicrobial resistance; ceftazidime; crystal structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pincus NB, Bachta KER, Ozer EA, Allen JP, Pura ON, Qi C, Rhodes NJ, Marty FM, Pandit A, Mekalanos JJ, Oliver A, Hauser AR. 2020. Long-term persistence of an extensively drug-resistant subclade of globally distributed Pseudomonas aeruginosa clonal complex 446 in an academic medical center. Clin Infect Dis 71:1524–1531. 10.1093/cid/ciz973. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NCI CCSG P30 CA060553/HHS | NIH | National Cancer Institute (NCI)
- R21 AI129167/AI/NIAID NIH HHS/United States
- U19 AI135964/AI/NIAID NIH HHS/United States
- T32 GM008061/GM/NIGMS NIH HHS/United States
- R01 AI053674/AI/NIAID NIH HHS/United States
- RO1 AI118257/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- GM118187/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- T32 GM008152/GM/NIGMS NIH HHS/United States
- R01 AI118257/AI/NIAID NIH HHS/United States
- U01 AI124316/AI/NIAID NIH HHS/United States
- HHSN272017000060C/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P30 CA060553/CA/NCI NIH HHS/United States
- P20 GM121176/GM/NIGMS NIH HHS/United States
- R35 GM118187/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

