Neoadjuvant Chemotherapy Is Associated with Altered Immune Cell Infiltration and an Anti-Tumorigenic Microenvironment in Resected Pancreatic Cancer
- PMID: 36129461
- PMCID: PMC9999119
- DOI: 10.1158/1078-0432.CCR-22-1125
Neoadjuvant Chemotherapy Is Associated with Altered Immune Cell Infiltration and an Anti-Tumorigenic Microenvironment in Resected Pancreatic Cancer
Abstract
Purpose: Neoadjuvant chemotherapy is increasingly administered to patients with resectable or borderline resectable pancreatic ductal adenocarcinoma (PDAC), yet its impact on the tumor immune microenvironment is incompletely understood.
Design: We employed quantitative, spatially resolved multiplex immunofluorescence and digital image analysis to identify T-cell subpopulations, macrophage polarization states, and myeloid cell subpopulations in a multi-institution cohort of up-front resected primary tumors (n = 299) and in a comparative set of resected tumors after FOLFIRINOX-based neoadjuvant therapy (n = 36) or up-front surgery (n = 30). Multivariable-adjusted Cox proportional hazards models were used to evaluate associations between the immune microenvironment and patient outcomes.
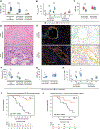
Results: In the multi-institutional resection cohort, immune cells exhibited substantial heterogeneity across patient tumors and were located predominantly in stromal regions. Unsupervised clustering using immune cell densities identified four main patterns of immune cell infiltration. One pattern, seen in 20% of tumors and characterized by abundant T cells (T cell-rich) and a paucity of immunosuppressive granulocytes and macrophages, was associated with improved patient survival. Neoadjuvant chemotherapy was associated with a higher CD8:CD4 ratio, greater M1:M2-polarized macrophage ratio, and reduced CD15+ARG1+ immunosuppressive granulocyte density. Within neoadjuvant-treated tumors, 72% showed a T cell-rich pattern with low immunosuppressive granulocytes and macrophages. M1-polarized macrophages were located closer to tumor cells after neoadjuvant chemotherapy, and colocalization of M1-polarized macrophages and tumor cells was associated with greater tumor pathologic response and improved patient survival.
Conclusions: Neoadjuvant chemotherapy with FOLFIRINOX shifts the PDAC immune microenvironment toward an anti-tumorigenic state associated with improved patient survival.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Hu Q, Wang D, Chen Y, Li X, Cao P, Cao D. Network meta-analysis comparing neoadjuvant chemoradiation, neoadjuvant chemotherapy and upfront surgery in patients with resectable, borderline resectable, and locally advanced pancreatic ductal adenocarcinoma. Radiat Oncol. 2019;14(1):1–8. doi: 10.1186/s13014-019-1330-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

