Selective Cerebrospinal Fluid Hypothermia: Bioengineering Development and In Vivo Study of an Intraventricular Cooling Device (V-COOL)
- PMID: 36129603
- PMCID: PMC9723013
- DOI: 10.1007/s13311-022-01302-y
Selective Cerebrospinal Fluid Hypothermia: Bioengineering Development and In Vivo Study of an Intraventricular Cooling Device (V-COOL)
Abstract
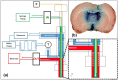
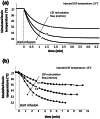
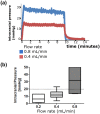
Hypothermia is a promising therapeutic strategy for severe vasospasm and other types of non-thrombotic cerebral ischemia, but its clinical application is limited by significant systemic side effects. We aimed to develop an intraventricular device for the controlled cooling of the cerebrospinal fluid, to produce a targeted hypothermia in the affected cerebral hemisphere with a minimal effect on systemic temperature. An intraventricular cooling device (acronym: V-COOL) was developed by in silico modelling, in vitro testing, and in vivo proof-of-concept application in healthy Wistar rats (n = 42). Cerebral cortical temperature, rectal temperature, and intracranial pressure were monitored at increasing flow rate (0.2 to 0.8 mL/min) and duration of application (10 to 60 min). Survival, neurological outcome, and MRI volumetric analysis of the ventricular system were assessed during the first 24 h. The V-COOL prototyping was designed to minimize extra-cranial heat transfer and intra-cranial pressure load. In vivo application of the V-COOL device produced a flow rate-dependent decrease in cerebral cortical temperature, without affecting systemic temperature. The target degree of cerebral cooling (- 3.0 °C) was obtained in 4.48 min at the flow rate of 0.4 mL/min, without significant changes in intracranial pressure. Survival and neurological outcome at 24 h showed no significant difference compared to sham-treated rats. MRI study showed a transient dilation of the ventricular system (+ 38%) in a subset of animals. The V-COOL technology provides an effective, rapid, selective, and safe cerebral cooling to a clinically relevant degree of - 3.0 °C.
Keywords: Cerebral ischemia; Cerebrospinal fluid; Device; Hypothermia; Neuroprotection; Vasospasm.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests. The V-COOL technology is not currently patent pending by any of the authors.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

