Economic evaluation of the Hepatitis C virus elimination program in the country of Georgia, 2015 to 2017
- PMID: 36129625
- PMCID: PMC10227952
- DOI: 10.1111/liv.15431
Economic evaluation of the Hepatitis C virus elimination program in the country of Georgia, 2015 to 2017
Abstract
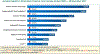
Background and aims: In 2015, the country of Georgia launched an elimination program aiming to reduce the prevalence of Hepatitis C virus (HCV) infection by 90% from 5.4% prevalence (~150 000 people). During the first 2.5 years of the program, 770 832 people were screened, 48 575 were diagnosed with active HCV infection, and 41 483 patients were treated with direct-acting antiviral (DAA)-based regimens, with a >95% cure rate.
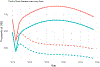
Methods: We modelled the incremental cost-effectiveness ratio (ICER) of HCV screening, diagnosis and treatment between April 2015 and November 2017 compared to no treatment, in terms of cost per quality-adjusted life year (QALY) gained in 2017 US dollars, with a 3% discount rate over 25 years. We compared the ICER to willingness-to-pay (WTP) thresholds of US$4357 (GDP) and US$871 (opportunity cost) per QALY gained.
Results: The average cost of screening, HCV viremia testing, and treatment per patient treated was $386 to the provider, $225 to the patient and $1042 for generic DAAs. At 3% discount, 0.57 QALYs were gained per patient treated. The ICER from the perspective of the provider including generic DAAs was $2285 per QALY gained, which is cost-effective at the $4357 WTP threshold, while if patient costs are included, it is just above the threshold at $4398/QALY. All other scenarios examined in sensitivity analyses remain cost-effective except for assuming a shorter time horizon to the end of 2025 or including the list price DAA cost. Reducing or excluding DAA costs reduced the ICER below the opportunity-cost WTP threshold.
Conclusions: The Georgian HCV elimination program provides valuable evidence that national programs for scaling up HCV screening and treatment for achieving HCV elimination can be cost-effective.
© 2022 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
PV and JGW received an investigator-sponsored research grant from Gilead Sciences, which partly contributed to this work. All authors declare no other conflict of interest.
Figures
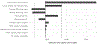
Similar articles
-
Cost-effectiveness of integrated treatment for hepatitis C virus (HCV) among people who inject drugs in Norway: An economic evaluation of the INTRO-HCV trial.Addiction. 2023 Dec;118(12):2424-2439. doi: 10.1111/add.16305. Epub 2023 Jul 29. Addiction. 2023. PMID: 37515462 Free PMC article.
-
Economic evaluation of pan-genotypic generic direct-acting antiviral regimens for treatment of chronic hepatitis C in Iran: a cost-effectiveness study.BMJ Open. 2022 Jun 8;12(6):e058757. doi: 10.1136/bmjopen-2021-058757. BMJ Open. 2022. PMID: 35676019 Free PMC article.
-
Cost-effectiveness of mass screening for Hepatitis C virus among all inmates in an Irish prison.Int J Drug Policy. 2021 Oct;96:103394. doi: 10.1016/j.drugpo.2021.103394. Epub 2021 Aug 17. Int J Drug Policy. 2021. PMID: 34412938 Free PMC article.
-
Systematic review: economic evaluations of HCV screening in the direct-acting antivirals era.Aliment Pharmacol Ther. 2019 May;49(9):1126-1133. doi: 10.1111/apt.15201. Epub 2019 Mar 6. Aliment Pharmacol Ther. 2019. PMID: 30843268
-
Cost-utility analysis of second-generation direct-acting antivirals for hepatitis C: a systematic review.Expert Rev Gastroenterol Hepatol. 2018 Dec;12(12):1251-1263. doi: 10.1080/17474124.2018.1540929. Epub 2018 Oct 31. Expert Rev Gastroenterol Hepatol. 2018. PMID: 30791790
Cited by
-
Cost-effectiveness of Hepatitis C virus self-testing in four settings.PLOS Glob Public Health. 2023 Apr 5;3(4):e0001667. doi: 10.1371/journal.pgph.0001667. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37018166 Free PMC article.
-
Modelling the impact and cost-effectiveness of non-governmental organizations on HIV and HCV transmission among people who inject drugs in Ukraine.J Int AIDS Soc. 2023 Apr;26(4):e26073. doi: 10.1002/jia2.26073. J Int AIDS Soc. 2023. PMID: 37012669 Free PMC article.
-
Cost-Effectiveness of Hepatitis C Virus Case Finding and Treatment in Eastern Europe and Central Asia.Liver Int. 2025 Aug;45(8):e70199. doi: 10.1111/liv.70199. Liver Int. 2025. PMID: 40600572 Free PMC article.
References
-
- World Health Organization Global Hepatitis Report 2017. World Health Organization; 2017. Accessed March 30, 2022. https://apps.who.int/iris/handle/10665/255016
-
- WHO-HIV-2016.06-eng.pdf. Accessed March 30, 2022. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-...
-
- Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accessed March 31, 2022. https://www.who.int/publications-detail-redirect/9789240027077
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

