Efficacy of MyPEEPS Mobile, an HIV Prevention Intervention Using Mobile Technology, on Reducing Sexual Risk Among Same-Sex Attracted Adolescent Males: A Randomized Clinical Trial
- PMID: 36129712
- PMCID: PMC9494195
- DOI: 10.1001/jamanetworkopen.2022.31853
Efficacy of MyPEEPS Mobile, an HIV Prevention Intervention Using Mobile Technology, on Reducing Sexual Risk Among Same-Sex Attracted Adolescent Males: A Randomized Clinical Trial
Abstract
Importance: HIV transmission rates in the United States have increased among men who have sex with men. However, there are no published randomized trials examining interventions to reduce sexual risk for HIV acquisition in males younger than 18 years.
Objective: To determine the efficacy of MyPEEPS Mobile, a mobile-delivered HIV prevention intervention, to reduce sexual risk behavior in same-sex attracted young males.
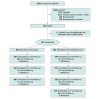
Design, setting, and participants: This was a national randomized clinical trial of the efficacy MyPEEPS Mobile vs a delayed intervention among males aged 13 to 18 years to prevent or reduce sexual risk for HIV acquisition. Study activities were completed through 4 study sites in Birmingham, Alabama; New York, New York; Seattle, Washington; and Chicago, Illinois. Study staff at each site met with participants in person or via video conferencing. Data were collected from June 1, 2018, to April 7, 2020, and analyzed from July to October 2021.
Interventions: The MyPEEPS Mobile intervention contains 21 online psychoeducational and skill-building modules, which participants completed over a 3-month period. Participants randomized to the intervention group received access to MyPEEPS Mobile for the first 3 months, while those randomized to the delayed intervention group received access at their 9-month visit after data for the primary efficacy analysis had been collected.
Main outcomes and measures: The self-reported primary outcome was change in the number of condomless anal sex acts between study conditions. Secondary outcomes were change in the number of sex partners, number of condomless anal sex partners, the number of sex acts while under the influence of substances, preexposure prophylactic uptake, nonoccupational postexposure prophylaxis use, and HIV and sexually transmitted infection testing.
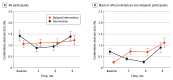
Results: In the analytic sample of 763 racially and ethnically diverse study participants, the mean (SD) age was 16.2 (1.4) years; 736 participants (97%) were male, 13 (2%) nonbinary; and 6 (1%) genderqueer; 158 (21%) were Black or African American, 311 (41%) were Hispanic or Latino, and 284 (37%) were White. Overall, 382 were randomized to the intervention group and 381 to the delayed intervention group. At 3-month follow-up, there was a significant reduction in the number of condomless anal sex acts in the intervention group compared with the delayed intervention group (incidence rate ratio [IRR], 0.56; 95% CI, 0.32-0.99); however, there was no significant difference between groups at 6 or 9 months. In subgroup analyses, the intervention effect was pronounced among Black non-Hispanic participants at 3-month follow-up (IRR, 0.19; 95% CI, 0.04-0.94) and 6-month follow-up (IRR, 0.15; 95% CI, 0.03-0.78) compared with the delayed intervention group. There were no significant differences in the change in the number of sex partners, number of condomless anal sex partners, the number of sex acts while under the influence of substances, preexposure prophylactic uptake, nonoccupational postexposure prophylaxis use, and HIV and sexually transmitted infection testing between the intervention and delayed intervention groups.
Conclusions and relevance: In this study, the MyPEEPS Mobile intervention demonstrated a 44% overall reduction in condomless anal sex at 3-month follow-up compared with the delayed intervention group, but not at 6 or 9 months. To our knowledge, MyPEEPS Mobile is the first intervention to demonstrate evidence of short-term efficacy for reducing sexual risk among same-sex attracted young males.
Trial registration: ClinicalTrials.gov Identifier: NCT03167606.
Conflict of interest statement
Figures
Similar articles
-
A randomized controlled efficacy trial of an mHealth HIV prevention intervention for sexual minority young men: MyPEEPS mobile study protocol.BMC Public Health. 2020 Jan 15;20(1):65. doi: 10.1186/s12889-020-8180-4. BMC Public Health. 2020. PMID: 31941475 Free PMC article.
-
Efficacy of an Empowerment-Based, Group-Delivered HIV Prevention Intervention for Young Transgender Women: The Project LifeSkills Randomized Clinical Trial.JAMA Pediatr. 2018 Oct 1;172(10):916-923. doi: 10.1001/jamapediatrics.2018.1799. JAMA Pediatr. 2018. PMID: 30105381 Free PMC article. Clinical Trial.
-
A Pilot Randomized Controlled Trial of an Integrated In-person and Mobile Phone Delivered Counseling and Text Messaging Intervention to Reduce HIV Transmission Risk among Male Sex Workers in Chennai, India.AIDS Behav. 2017 Nov;21(11):3172-3181. doi: 10.1007/s10461-017-1884-5. AIDS Behav. 2017. PMID: 28831618 Free PMC article. Clinical Trial.
-
Randomized Controlled Trials of Technology-Based HIV/STI and Drug Abuse Preventive Interventions for African American and Hispanic Youth: Systematic Review.JMIR Public Health Surveill. 2017 Dec 13;3(4):e96. doi: 10.2196/publichealth.7129. JMIR Public Health Surveill. 2017. PMID: 29237577 Free PMC article. Review.
-
A computational future for preventing HIV in minority communities: how advanced technology can improve implementation of effective programs.J Acquir Immune Defic Syndr. 2013 Jun 1;63 Suppl 1(0 1):S72-84. doi: 10.1097/QAI.0b013e31829372bd. J Acquir Immune Defic Syndr. 2013. PMID: 23673892 Free PMC article. Review.
Cited by
-
A feasibility study of the use of HIV self-tests in young men who have sex with men.AIDS Care. 2023 Sep;35(9):1279-1284. doi: 10.1080/09540121.2022.2160864. Epub 2023 Jan 6. AIDS Care. 2023. PMID: 36608217 Free PMC article.
-
Evaluating the Effectiveness of a Mobile Health Intervention on Enhancing HIV Knowledge in Sexual and Gender Minority Men.J Acquir Immune Defic Syndr. 2025 Mar 1;98(3):217-223. doi: 10.1097/QAI.0000000000003562. J Acquir Immune Defic Syndr. 2025. PMID: 39813261 Free PMC article. Clinical Trial.
-
Feasibility of the Adapted MyPEEPS Mobile App for HIV Prevention in Young Transgender Men.AIDS Educ Prev. 2025 Jul;37(3):173-186. doi: 10.1521/aeap.2025.37.3.173. AIDS Educ Prev. 2025. PMID: 40577394 Free PMC article. Clinical Trial.
-
Use of Behavior Change Techniques in Digital HIV Prevention Programs for Adolescents and Young People: Systematic Review.JMIR Public Health Surveill. 2025 Apr 28;11:e59519. doi: 10.2196/59519. JMIR Public Health Surveill. 2025. PMID: 40293783 Free PMC article.
-
User preferences for an mHealth app to support HIV testing and pre-exposure prophylaxis uptake among men who have sex with men in Malaysia.PLOS Digit Health. 2024 Oct 30;3(10):e0000643. doi: 10.1371/journal.pdig.0000643. eCollection 2024 Oct. PLOS Digit Health. 2024. PMID: 39475851 Free PMC article.
References
-
- US Centers for Disease Control and Prevention . HIV among gay and bisexual men. Reviewed June 28, 2022. Accessed August 17, 2022. https://www.cdc.gov/hiv/group/msm/index.html
-
- US Department of Health & Human Services . US statistics: fast facts. Accessed August 17, 2022. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics
-
- US Centers for Disease Control and Prevention . CDC Fact Sheet: HIV among gay and bisexual men. Accessed August 17, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous