Effectiveness of Standard Sequential Bilateral Repetitive Transcranial Magnetic Stimulation vs Bilateral Theta Burst Stimulation in Older Adults With Depression: The FOUR-D Randomized Noninferiority Clinical Trial
- PMID: 36129719
- PMCID: PMC9494264
- DOI: 10.1001/jamapsychiatry.2022.2862
Effectiveness of Standard Sequential Bilateral Repetitive Transcranial Magnetic Stimulation vs Bilateral Theta Burst Stimulation in Older Adults With Depression: The FOUR-D Randomized Noninferiority Clinical Trial
Abstract
Importance: Treatment-resistant depression (TRD) is common in older adults. Bilateral repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex for 48 minutes has demonstrated efficacy in TRD. Theta burst stimulation (TBS), a newer form of rTMS, can also be delivered bilaterally using left intermittent TBS and right continuous TBS for only 4 minutes.
Objective: To establish the effectiveness and tolerability of TBS compared with standard rTMS in older adults with TRD.
Design, setting, and participants: In this randomized noninferiority trial with open treatment and blinded assessors, recruitment occurred between December 2016 and March 2020. The trial was conducted at the Centre for Addiction and Mental Health in Toronto, Ontario, Canada and included outpatients 60 years and older with a diagnosis of depression, moderate severity, and nonresponse to 1 or more antidepressant trial of adequate dosage and duration or intolerance of 2 or more trials.
Interventions: Participants were randomized to receive a course of 4 to 6 weeks of either bilateral standard rTMS or TBS.
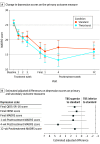
Main outcomes and measures: The primary outcome measure was change in Montgomery-Åsberg Depression Rating Scale; secondary outcome measures included the 17-item Hamilton Rating Scale for Depression, Quick Inventory of Depressive Symptomatology (16-item) (self-report), and dropout rates. A noninferiority margin of 2.75 points was used for the primary outcome. All participants who attained the primary completion point of 4 weeks were analyzed.
Results: A total of 87 participants (mean [SD] age, 67.1 [6.7] years; 47 [54.0%] female) were randomized to standard bilateral rTMS and 85 (mean [SD] age, 66.3 [5.3] years; 45 [52.9%] female) to TBS, of whom 85 (98%) and 79 (93%) were assessed for the primary outcome, respectively, whereas tolerability was assessed in all randomized participants. In the rTMS group, 4 (4.6%) were American Indian, reported other, or preferred not to answer; 5 (5.8%) were Asian; and 78 (89.7%) were White. In the TBS group, 6 (7.1%) were Asian, 2 (2.4%) were Black or reported other, and 77 (90.3%) were White. Mean (SD) Montgomery-Åsberg Depression Rating Scale total scores improved from 25.6 (4.0) to 17.3 (8.9) for rTMS and 25.7 (4.7) to 15.8 (9.1) for TBS (adjusted difference, 1.55; lower 95% CI -0.67), establishing noninferiority for TBS. The all-cause dropout rates were relatively similar between groups (rTMS: 2 of 87 [2.3%]; TBS: 6 of 85 [7.1%]; P = .14; χ2 = 2.2).
Conclusions and relevance: In older adults with TRD, bilateral TBS compared with standard bilateral rTMS achieved noninferior reduction in depression symptoms. Both treatments had low and similar dropout rates. Using TBS rather than rTMS could increase access to treatment several-fold for older adults with TRD.
Trial registration: ClinicalTrials.gov Identifier: NCT02998580.
Conflict of interest statement
Figures

References
-
- McClintock SM, Reti IM, Carpenter LL, et al. ; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments . Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1):16cs10905. doi:10.4088/JCP.16cs10905 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

