Pain Reduction With an Immersive Digital Therapeutic Tool in Women Living With Endometriosis-Related Pelvic Pain: Randomized Controlled Trial
- PMID: 36129733
- PMCID: PMC9536521
- DOI: 10.2196/39531
Pain Reduction With an Immersive Digital Therapeutic Tool in Women Living With Endometriosis-Related Pelvic Pain: Randomized Controlled Trial
Abstract
Background: Chronic pelvic pain is a common and disabling condition in women living with endometriosis. Pharmacological and surgical treatments are not always effective at controlling pain and present important restrictions. Digital therapeutics (DTx) are emerging as major nonpharmacological alternatives that aim to extend the analgesic therapeutic arsenal of patients.
Objective: In this randomized controlled trial (RCT), we aimed to measure the immediate and 4-hour persisting effects of a single use 20-minute DTx (Endocare) on pain in women experiencing pelvic pain due to endometriosis.
Methods: A total of 45 women with endometriosis participated in a randomized controlled study comparing the analgesic effect of a single use of a virtual reality digital treatment named Endocare (n=23, 51%) to a 2D digital control (n=22, 49%). Perceived pain and pain relief were measured before the treatment and 15, 30, 45, 60, and 240 minutes after the end of the treatment.
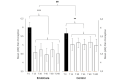
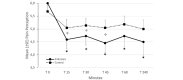
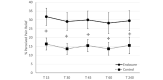
Results: The clustered posttreatment pain was significantly reduced compared to the pretreatment for both Endocare and the control group (all P<.01). Endocare was significantly more effective than the control group (all P<.01). Endocare decreased the mean pain intensity from 6.0 (SD 1.31) before the treatment to 4.5 (SD 1.71) posttreatment, while the control only decreased it from 5.7 (SD 1.36) to 5.0 (SD 1.43). When comparing each posttreatment measures to the pretest, Endocare significantly reduced pain perception for all points in time up to 4 hours posttreatment. The differences did not reached significance for the control group. Moreover, Endocare was significantly superior to the control group 15, 30, and 45 minutes after the treatment (all P<.001). The mean perceived pain relief was significantly higher for Endocare at 28% (SD 2%) compared to the control, which was 15% (SD 1%) for all the posttreatment measurements (all P>.05).
Conclusions: Our study aimed to test the effects of a single use of a DTx treatment on reported pain at different time points in women diagnosed with endometriosis experiencing moderate-to-severe pelvic pain. Importantly, our results support that Endocare, a virtual reality immersive treatment, significantly reduce pain perception compared to a digital control in women living with endometriosis. Interestingly, we are the first to notice that the effect persisted up to 4 hours posttreatment.
Trial registration: ClinicalTrials.gov NCT04650516; https://tinyurl.com/2a2eu9wv.
Keywords: RCT; chronic pain; digital health; digital intervention; digital therapeutics; digital treatment; eHealth; effectiveness; efficacy; endometriosis; endometrium; gynecologist; gynecology; pain; pelvic; pelvic pain; pelvis; randomized controlled trial; reproductive health; sexual health; virtual reality; women's health.
©Benjamin Merlot, Garance Dispersyn, Zoé Husson, Isabella Chanavaz-Lacheray, Thomas Dennis, Juliette Greco-Vuilloud, Maxime Fougère, Stéphane Potvin, Maryne Cotty-Eslous, Horace Roman, Serge Marchand. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 21.09.2022.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Pain Reduction With an Immersive Digital Therapeutic in Women Living With Endometriosis-Related Pelvic Pain: At-Home Self-Administered Randomized Controlled Trial.J Med Internet Res. 2023 Jun 28;25:e47869. doi: 10.2196/47869. J Med Internet Res. 2023. PMID: 37260160 Free PMC article. Clinical Trial.
-
A Single Session of a Digital Health Tool-Delivered Exercise Intervention May Provide Immediate Relief from Pelvic Pain in Women with Endometriosis: A Pilot Randomized Controlled Study.Int J Environ Res Public Health. 2023 Jan 17;20(3):1665. doi: 10.3390/ijerph20031665. Int J Environ Res Public Health. 2023. PMID: 36767032 Free PMC article. Clinical Trial.
-
Japanese-style acupuncture for endometriosis-related pelvic pain in adolescents and young women: results of a randomized sham-controlled trial.J Pediatr Adolesc Gynecol. 2008 Oct;21(5):247-57. doi: 10.1016/j.jpag.2007.07.008. J Pediatr Adolesc Gynecol. 2008. PMID: 18794019 Free PMC article. Clinical Trial.
-
Pre- and postsurgical medical therapy for endometriosis surgery.Cochrane Database Syst Rev. 2020 Nov 18;11(11):CD003678. doi: 10.1002/14651858.CD003678.pub3. Cochrane Database Syst Rev. 2020. PMID: 33206374 Free PMC article.
-
An evidence-based medicine approach to the treatment of endometriosis-associated chronic pelvic pain: placebo-controlled studies.J Am Assoc Gynecol Laparosc. 2000 Nov;7(4):477-88. doi: 10.1016/s1074-3804(05)60360-x. J Am Assoc Gynecol Laparosc. 2000. PMID: 11044498 Review.
Cited by
-
Sensory stimulations potentializing digital therapeutics pain control.Front Pain Res (Lausanne). 2023 Sep 6;4:1168377. doi: 10.3389/fpain.2023.1168377. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37745799 Free PMC article. Review.
-
Pain Reduction With an Immersive Digital Therapeutic in Women Living With Endometriosis-Related Pelvic Pain: At-Home Self-Administered Randomized Controlled Trial.J Med Internet Res. 2023 Jun 28;25:e47869. doi: 10.2196/47869. J Med Internet Res. 2023. PMID: 37260160 Free PMC article. Clinical Trial.
-
Efficacy of virtual reality for pain relief in medical procedures: a systematic review and meta-analysis.BMC Med. 2024 Feb 14;22(1):64. doi: 10.1186/s12916-024-03266-6. BMC Med. 2024. PMID: 38355563 Free PMC article.
-
Association between depression and endometriosis using data from NHANES 2005-2006.Sci Rep. 2023 Oct 31;13(1):18708. doi: 10.1038/s41598-023-46005-2. Sci Rep. 2023. PMID: 37907559 Free PMC article.
-
An overview and comprehensive analysis of interdisciplinary clinical research in endometriosis based on trial registry.iScience. 2024 Feb 20;27(3):109298. doi: 10.1016/j.isci.2024.109298. eCollection 2024 Mar 15. iScience. 2024. PMID: 38455973 Free PMC article.
References
-
- Saunders PTK, Horne AW. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell. 2021 May 27;184(11):2807–2824. doi: 10.1016/j.cell.2021.04.041. https://linkinghub.elsevier.com/retrieve/pii/S0092-8674(21)00576-6 S0092-8674(21)00576-6 - DOI - PubMed
-
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010 Jun 24;362(25):2389–98. doi: 10.1056/NEJMcp1000274. http://europepmc.org/abstract/MED/20573927 362/25/2389 - DOI - PMC - PubMed
-
- Tirlapur S A, Kuhrt K, Chaliha C, Ball E, Meads C, Khan K S. The 'evil twin syndrome' in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013 May;11(3):233–7. doi: 10.1016/j.ijsu.2013.02.003. https://linkinghub.elsevier.com/retrieve/pii/S1743-9191(13)00034-4 S1743-9191(13)00034-4 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

