HIV-1 Vpr suppresses expression of the thiazide-sensitive sodium chloride co-transporter in the distal convoluted tubule
- PMID: 36129874
- PMCID: PMC9491550
- DOI: 10.1371/journal.pone.0273313
HIV-1 Vpr suppresses expression of the thiazide-sensitive sodium chloride co-transporter in the distal convoluted tubule
Abstract
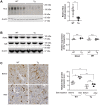
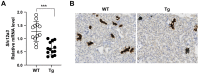
HIV-associated nephropathy (HIVAN) impairs functions of both glomeruli and tubules. Attention has been previously focused on the HIVAN glomerulopathy. Tubular injury has drawn increased attention because sodium wasting is common in hospitalized HIV/AIDS patients. We used viral protein R (Vpr)-transgenic mice to investigate the mechanisms whereby Vpr contributes to urinary sodium wasting. In phosphoenolpyruvate carboxykinase promoter-driven Vpr-transgenic mice, in situ hybridization showed that Vpr mRNA was expressed in all nephron segments, including the distal convoluted tubule. Vpr-transgenic mice, compared with wild-type littermates, markedly increased urinary sodium excretion, despite similar plasma renin activity and aldosterone levels. Kidneys from Vpr-transgenic mice also markedly reduced protein abundance of the Na+-Cl- cotransporter (NCC), while mineralocorticoid receptor (MR) protein expression level was unchanged. In African green monkey kidney cells, Vpr abrogated the aldosterone-mediated stimulation of MR transcriptional activity. Gene expression of Slc12a3 (NCC) in Vpr-transgenic mice was significantly lower compared with wild-type mice, assessed by both qRT-PCR and RNAScope in situ hybridization analysis. Chromatin immunoprecipitation assays identified multiple MR response elements (MRE), located from 5 kb upstream of the transcription start site and extending to the third exon of the SLC12A3 gene. Mutation of MRE and SP1 sites in the SLC12A3 promoter region abrogated the transcriptional responses to aldosterone and Vpr, indicating that functional MRE and SP1 are required for the SLC12A3 gene suppression in response to Vpr. Thus, Vpr attenuates MR transcriptional activity and inhibits Slc12a3 transcription in the distal convoluted tubule and contributes to salt wasting in Vpr-transgenic mice.
Conflict of interest statement
Author JBK holds a patent relating to monoclonal antibodies to HIV-1 Vpr and methods of using same. United States Patent 7,993,647 (2015). No other conflicts of interest, financial or otherwise, are declared by the authors. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

