Associations of Immunogenicity and Reactogenicity After Severe Acute Respiratory Syndrome Coronavirus 2 mRNA-1273 Vaccine in the COVE and TeenCOVE Trials
- PMID: 36130187
- PMCID: PMC10202429
- DOI: 10.1093/cid/ciac780
Associations of Immunogenicity and Reactogenicity After Severe Acute Respiratory Syndrome Coronavirus 2 mRNA-1273 Vaccine in the COVE and TeenCOVE Trials
Abstract
Background: The reactogenicity and immunogenicity of coronavirus disease 2019 (COVID-19) vaccines are well studied. Little is known regarding the relationship between immunogenicity and reactogenicity of COVID-19 vaccines.
Methods: This study assessed the association between immunogenicity and reactogenicity after 2 mRNA-1273 (100 µg) injections in 1671 total adolescent and adult participants (≥12 years) from the primary immunogenicity sets of the blinded periods of the Coronavirus Efficacy (COVE) and TeenCOVE trials. Associations between immunogenicity through day 57 and solicited adverse reactions (ARs) after the first and second injections of mRNA-1273 were evaluated among participants with and without solicited ARs using linear mixed-effects models.
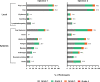
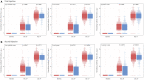
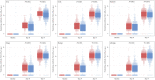
Results: mRNA-1273 reactogenicity in this combined analysis set was similar to that reported for these trials. The vaccine elicited high neutralizing antibody (nAb) geometric mean titers (GMTs) in evaluable participants. GMTs at day 57 were significantly higher in participants who experienced solicited systemic ARs after the second injection (1227.2 [1164.4-1293.5]) than those who did not (980.1 [886.8-1083.2], P = .001) and were associated with fever, chills, headache, fatigue, myalgia, and arthralgia. Significant associations with local ARs were not found.
Conclusions: These data show an association of systemic ARs with increased nAb titers following a second mRNA-1273 injection. While these data indicate systemic ARs are associated with increased antibody titers, high nAb titers were observed in participants after both injections, consistent with the immunogenicity and efficacy in these trials. These results add to the body of evidence regarding the relationship of immunogenicity and reactogenicity and can contribute toward the design of future mRNA vaccines.
Keywords: SARS-CoV-2; immunogenicity; mRNA-1273; reactogenicity; solicited adverse reactions.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Moderna, Inc . The authorization or approval status of Moderna COVID 19 vaccine in the U.S. and other countries. Available at:https://modernacovid19global.com/en-US. Accessed 15 August 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

