Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Pregnancy in Sub-Saharan Africa: A 6-Country Retrospective Cohort Analysis
- PMID: 36130257
- PMCID: PMC9214158
- DOI: 10.1093/cid/ciac294
Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Pregnancy in Sub-Saharan Africa: A 6-Country Retrospective Cohort Analysis
Abstract
Background: Few data are available on COVID-19 outcomes among pregnant women in sub-Saharan Africa (SSA), where high-risk comorbidities are prevalent. We investigated the impact of pregnancy on SARS-CoV-2 infection and of SARS-CoV-2 infection on pregnancy to generate evidence for health policy and clinical practice.
Methods: We conducted a 6-country retrospective cohort study among hospitalized women of childbearing age between 1 March 2020 and 31 March 2021. Exposures were (1) pregnancy and (2) a positive SARS-CoV-2 RT-PCR test. The primary outcome for both analyses was intensive care unit (ICU) admission. Secondary outcomes included supplemental oxygen requirement, mechanical ventilation, adverse birth outcomes, and in-hospital mortality. We used log-binomial regression to estimate the effect between pregnancy and SARS-CoV-2 infection. Factors associated with mortality were evaluated using competing-risk proportional subdistribution hazards models.
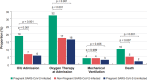
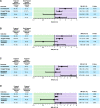
Results: Our analyses included 1315 hospitalized women: 510 pregnant women with SARS-CoV-2, 403 nonpregnant women with SARS-CoV-2, and 402 pregnant women without SARS-CoV-2 infection. Among women with SARS-CoV-2 infection, pregnancy was associated with increased risk for ICU admission (adjusted risk ratio [aRR]: 2.38; 95% CI: 1.42-4.01), oxygen supplementation (aRR: 1.86; 95% CI: 1.44-2.42), and hazard of in-hospital death (adjusted sub-hazard ratio [aSHR]: 2.00; 95% CI: 1.08-3.70). Among pregnant women, SARS-CoV-2 infection increased the risk of ICU admission (aRR: 2.0; 95% CI: 1.20-3.35), oxygen supplementation (aRR: 1.57; 95% CI: 1.17-2.11), and hazard of in-hospital death (aSHR: 5.03; 95% CI: 1.79-14.13).
Conclusions: Among hospitalized women in SSA, both SARS-CoV-2 infection and pregnancy independently increased risks of ICU admission, oxygen supplementation, and death. These data support international recommendations to prioritize COVID-19 vaccination among pregnant women.
Keywords: Africa; COVID-19; maternal; neonate; pregnancy.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. B. N. is an infectious disease internist and epidemiologist and Principal Investigator (PI) of NIH/FIC grant numbers 1R25TW011217-01, 1R21TW011706-01, and 1D43TW010937-01A1; and payment to University of Pittsburgh. N. A. S.-A. is a clinician-scientist in Pediatric Infectious Diseases and implementation research; she is supported by the NIH National Institute of Child Health and Human Development (NICHD) grant number R01HD089866; by an NIH/FIC award through the Adolescent HIV Prevention and Treatment Implementation Science Alliance (AHISA) for the Central and West Africa Implementation Science Alliance (CAWISA) (payment to institution); and by NIH/FIC grant number 1D43TW012280-01. F. S., N. K. S., and A. P. are supported as PIs by NIH/FIC grant number 1R25TW011217-01. A. Z. is PIs of the Pan-African Network on Emerging and Re-Emerging Infections (PANDORA-ID-NET; https://www.pandora-id.net) funded by the European Developing Countries Clinical Trial Partnership (EDCTP) and the European Union Horizon 2020 Framework Program for Research and Innovation, paid to institution (University College London, London, UK). R. N. M. reports grants or contracts unrelated to this work, and payment to their institution: NIH/FIC1D43TW010547-01—The African Center for Biostatistical Excellence (ACBE). P. J. R. reports the following grants or contracts unrelated to this work and paid to their institution: 5R01AI075045-12, 5R01AI139179-04, and 5R01AI117001-07. E. R. S. reports a Bill and Melinda Gates Foundation grant for a prospective meta-analysis of COVID-19 in pregnancy, unrelated to this work, and payment to George Washington University. A. B. reports grants or contracts unrelated to this work—NIH: IMPAACT Vice-chair Funding for P1106; and UNITAID: Benefit KIDS funding for PETITE Study. P. A. reports grants or contracts unrelated to this work and paid to the University of Ibadan, Nigeria: NIH/FIC R25 grant number 1R25TW011217-01 to AFREhealth. J. W. N. reports grants paid to the University of Pittsburgh from the NIH to the Pitt-Ohio State Clinical Trials Unit (UM1 AI068636), the University of Pittsburgh Virology Support Laboratory (UM1 AI106701), the I4C Martin Delaney Collaboratory for an HIV Cure (UM1 AI126603), the REACH Martin Delaney Collaboratory for HIV Cure (UM1 AI164565), and from the National Cancer Institute through Leidos contract numbers HHSN261200800001E and 75N91019D00024 USAID; Gilead Sciences, Inc; and Janssen Pharmaceuticals. J. W. N. also reports consulting fees from Gilead Sciences, Inc (Scientific Advisory Board), Accelevir Diagnostics (Consulting Agreement), and Merck (Consulting Agreement); shares from Abound Bio, Inc, share options from Co-Crystal Pharma, Inc, and share options from Infectious Diseases Connect; a consulting agreement with Xi’an Yufan Biotechnologies; and employment with the University of Pittsburgh. M. S. reports grants or contracts unrelated to this work and paid to their institution (MGH-Boston, MA, USA): NIH/NIA R01AG059504-03 and NIH/NHLBI R01 HL141053-04. A.-P. K. reports research support unrelate to this work paid to their institution (South African Medical Research Council): NIH/FIC 1R21TW011706-01-Dolutegravir, Weight Gain and Metabolic Outcomes in South Africa. L. M. reports consulting fees from the World Health Organization on COVID in pregnancy and mother to child SARS-CoV-2 transmission (this contract is now completed); and payment from Virology Education for a talk on SARS-CoV-2 in pregnancy and possibility of mother-to-child SARS-CoV-2 transmission for continuing education sponsored by Virology Education. M. Y. is PI of the NIH/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant number 5U01AI096299-13 of the Central Africa-International epidemiology to Evaluate AIDS (CA-IeDEA). M. Y. also reports grants or contracts unrelated to this work and paid to their institution: NIH grant numbers 5U01AI096299 (NIAID), R01HD087993 (NICHD), U54CA254568 (National Cancer Institute), and R01HD105526 (NICHD). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

