SARS-CoV-2 Omicron BA.5: Evolving tropism and evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern
- PMID: 36130476
- PMCID: PMC9482529
- DOI: 10.1016/j.ebiom.2022.104270
SARS-CoV-2 Omicron BA.5: Evolving tropism and evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern
Abstract
Background: Genetically distinct viral variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been recorded since January 2020. The introduction of global vaccine programs has contributed to lower COVID-19 hospitalisation and mortality rates, particularly in developed countries. In late 2021, Omicron BA.1 emerged, with substantially altered genetic differences and clinical effects from other variants of concern. Shortly after dominating global spread in early 2022, BA.1 was supplanted by the genetically distinct Omicron lineage BA.2. A sub-lineage of BA.2, designated BA.5, presently has an outgrowth advantage over BA.2 and other BA.2 sub-lineages. Here we study the neutralisation of Omicron BA.1, BA.2 and BA.5 and pre-Omicron variants using a range of vaccine and convalescent sera and therapeutic monoclonal antibodies using a live virus neutralisation assay. Using primary nasopharyngeal swabs, we also tested the relative fitness of BA.5 compared to pre-Omicron and Omicron viral lineages in their ability to use the ACE2-TMPRSS2 pathway.
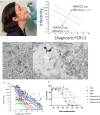
Methods: Using low passage clinical isolates of Clade A.2.2, Beta, Delta, BA.1, BA.2 and BA.5, we determined humoral neutralisation in vitro in vaccinated and convalescent cohorts, using concentrated human IgG pooled from thousands of plasma donors, and licensed monoclonal antibody therapies. We then determined infectivity to particle ratios in primary nasopharyngeal samples and expanded low passage isolates in a genetically engineered ACE2/TMPRSS2 cell line in the presence and absence of the TMPRSS2 inhibitor Nafamostat.
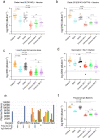
Findings: Peak responses to 3 doses of BNT162b2 vaccine were associated with a 9-fold reduction in neutralisation for Omicron lineages BA.1, BA.2 and BA.5. Concentrated pooled human IgG from convalescent and vaccinated donors and BNT162b2 vaccination with BA.1 breakthrough infections were associated with greater breadth of neutralisation, although the potency was still reduced 7-fold across all Omicron lineages. Testing of clinical grade antibodies revealed a 14.3-fold reduction using Evusheld and 16.8-fold reduction using Sotrovimab for the BA.5. Whilst the infectivity of BA.1 and BA.2 was attenuated in ACE2/TMPRSS2 entry, BA.5 was observed to be equivalent to that of an early 2020 circulating clade and had greater sensitivity to the TMPRSS2 inhibitor Nafamostat.
Interpretation: Observations support all Omicron variants to significantly escape neutralising antibodies across a range of vaccination and/or convalescent responses. Potency of therapeutic monoclonal antibodies is also reduced and differs across Omicron lineages. The key difference of BA.5 from other Omicron sub-variants is the reversion in tropism back to using the well-known ACE2-TMPRSS2 pathway, utilised efficiently by pre-Omicron lineages. Monitoring if these changes influence transmission and/or disease severity will be key for ongoing tracking and management of Omicron waves globally.
Funding: This work was primarily supported by Australian Medical Foundation research grants MRF2005760 (ST, GM & WDR), MRF2001684 (ADK and ST) and Medical Research Future Fund Antiviral Development Call grant (WDR), Medical Research Future Fund COVID-19 grant (MRFF2001684, ADK & SGT) and the New South Wales Health COVID-19 Research Grants Round 2 (SGT).
Keywords: ACE2; BA.2; BA.5; Neutralising antibodies; Omicron BA.1; SARS-CoV-2; TMPRSS2.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests A.O.S contributed to this manuscript in his capacity as adjunct associate lecturer (from January 2022) at the University of New South Wales. Since January 2022, A.O.S is an employee of GlaxoSmithKline Australia (medical science liaison for COVID-19).
Figures


References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

