ATP hydrolysis tunes specificity of a AAA+ protease
- PMID: 36130509
- PMCID: PMC9558560
- DOI: 10.1016/j.celrep.2022.111405
ATP hydrolysis tunes specificity of a AAA+ protease
Abstract
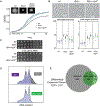
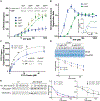
In bacteria, AAA+ proteases such as Lon and ClpXP degrade substrates with exquisite specificity. These machines capture the energy of ATP hydrolysis to power unfolding and degradation of target substrates. Here, we show that a mutation in the ATP binding site of ClpX shifts protease specificity to promote degradation of normally Lon-restricted substrates. However, this ClpX mutant is worse at degrading ClpXP targets, suggesting an optimal balance in substrate preference for a given protease that is easy to alter. In vitro, wild-type ClpXP also degrades Lon-restricted substrates more readily when ATP levels are reduced, similar to the shifted specificity of mutant ClpXP, which has altered ATP hydrolysis kinetics. Based on these results, we suggest that the rates of ATP hydrolysis not only power substrate unfolding and degradation, but also tune protease specificity. We consider various models for this effect based on emerging structures of AAA+ machines showing conformationally distinct states.
Keywords: AAA+ protease; ATP dependent proteases; CP: Molecular biology; Caulobacter; ClpX; ClpXP; Lon.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

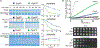


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

