Striatal dopamine explains novelty-induced behavioral dynamics and individual variability in threat prediction
- PMID: 36130595
- PMCID: PMC9671833
- DOI: 10.1016/j.neuron.2022.08.022
Striatal dopamine explains novelty-induced behavioral dynamics and individual variability in threat prediction
Abstract
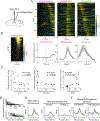
Animals both explore and avoid novel objects in the environment, but the neural mechanisms that underlie these behaviors and their dynamics remain uncharacterized. Here, we used multi-point tracking (DeepLabCut) and behavioral segmentation (MoSeq) to characterize the behavior of mice freely interacting with a novel object. Novelty elicits a characteristic sequence of behavior, starting with investigatory approach and culminating in object engagement or avoidance. Dopamine in the tail of the striatum (TS) suppresses engagement, and dopamine responses were predictive of individual variability in behavior. Behavioral dynamics and individual variability are explained by a reinforcement-learning (RL) model of threat prediction in which behavior arises from a novelty-induced initial threat prediction (akin to "shaping bonus") and a threat prediction that is learned through dopamine-mediated threat prediction errors. These results uncover an algorithmic similarity between reward- and threat-related dopamine sub-systems.
Keywords: avoidance; dopamine; initialization; neophobia; novelty; prediction; reinforcement learning; tail of the striatum; threat; threat prediction error; uncertainty.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





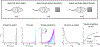

Comment in
-
Dopamine in the rodent tail of striatum regulates behavioral variability in response to threatening novel objects.Neuron. 2022 Nov 16;110(22):3653-3655. doi: 10.1016/j.neuron.2022.10.019. Neuron. 2022. PMID: 36395752
References
-
- Blanchard DC, Blanchard RJ, and Rodgers RJ (1991). Risk Assessment and Animal Models of Anxiety. In Animal Models in Psychopharmacology, Olivier B, Mos J, and Slangen JL, eds. (Basel: Birkhäuser; ), pp. 117–134.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

