Mortality and Heart Failure Hospitalization Among Young Adults With and Without Cardiogenic Shock After Acute Myocardial Infarction
- PMID: 36130688
- PMCID: PMC10403806
- DOI: 10.1016/j.cardfail.2022.08.012
Mortality and Heart Failure Hospitalization Among Young Adults With and Without Cardiogenic Shock After Acute Myocardial Infarction
Abstract
Objectives: To investigate risk factors and outcomes of cardiogenic shock complicating acute myocardial infarction (AMI-CS) in young patients with AMI.
Background: AMI-CS is associated with high morbidity and mortality rates. Data regarding AMI-CS in younger individuals are limited.
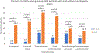
Methods and results: Consecutive patients with type 1 AMI aged 18-50 years admitted to 2 large tertiary-care academic centers were included, and they were adjudicated as having cardiogenic shock (CS) by physician review of electronic medical records using the Society for Cardiovascular Angiography and Interventions CS classification system. Outcomes included all-cause mortality (ACM), cardiovascular mortality (CVM) and 1-year hospitalization for heart failure (HHF). In addition to using the full population, matching was also used to define a comparator group in the non-CS cohort. Among 2097 patients (mean age 44 ± 5.1 years, 74% white, 19% female), AMI-CS was present in 148 (7%). Independent risk factors of AMI-CS included ST-segment elevation myocardial infarction, left main disease, out-of-hospital cardiac arrest, female sex, peripheral vascular disease, and diabetes. Over median follow-up of 11.2 years, young patients with AMI-CS had a significantly higher risk of ACM (adjusted HR 2.84, 95% CI 1.68-4.81; P < 0.001), CVM (adjusted HR 4.01, 95% CI 2.17-7.71; P < 0.001), and 1-year HHF (adjusted HR 5.99, 95% CI 2.04-17.61; P = 0.001) compared with matched non-AMI-CS patients. Over the course of the study, there was an increase in the incidence of AMI-CS among young patients with MI as well as rising mortality rates for patients with both AMI-CS and non-AMI-CS.
Conclusions: Of young patients with AMI, 7% developed AMI-CS, which was associated with a significantly elevated risk of mortality and HHF.
Keywords: Myocardial infarction; cardiogenic shock; heart failure hospitalization; mortality; risk factors; young.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures Sanjay Divakaran: Grant funding: Joint KL2/Catalyst Medical Research Investigator Training (CMeRIT) award from Harvard Catalyst and the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679-10). James Januzzi: Trustee, American College of Cardiology; Research Support/Funding, Applied Therapeutics, Innolife, Novartis Pharmaceuticals, and Abbott Diagnostics; Consulting: Abbott, Janssen, Novartis, and Roche Diagnostics; Clinical Endpoint Committees/Data Safety Monitoring Boards: Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, MyoKardia, and Takeda. Garrick Stewart: Consulting: Abbott Laboratories, Procyrion. Deepak L. Bhatt: Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, DRS.LINQ (stock options), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); site co-investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee, American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. Ron Blankstein: Research support: Amgen, Novartis; Consulting: Roivant Sciences, Caristo Diagnostics, Silence Therapeutics. All other authors have no disclosures.
Figures
Comment in
-
Acute Myocardial Infarction Cardiogenic Shock in Younger Adults: A Patient's Experience.J Card Fail. 2023 Jan;29(1):30-32. doi: 10.1016/j.cardfail.2022.12.001. Epub 2022 Dec 8. J Card Fail. 2023. PMID: 36496110 No abstract available.
References
-
- Aissaoui N, Puymirat E, Delmas C, Ortuno S, Durand E, Bataille V, et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail 2020;22:664–72. - PubMed
-
- De Luca L, Olivari Z, Farina A, Gonzini L, Lucci D, Di Chiara A, et al. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes: management changes in cardiogenic shock. Eur J Heart Fail 2015;17:1124–32. - PubMed
-
- Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. NRMI Investigators. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448. - PubMed
-
- Berg DD, Bohula EA, Morrow DA. Berg DD, Bohula EA, Morrow DA. Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care 2021;27:401–8. - PubMed
-
- Goldberg RJ, Samad NA, Yarzebski J, Gurwitz J, Bigelow C, Gore JM. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med 1999;340:1162–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

