Pain-autonomic measures reveal nociceptive sensitization in complex regional pain syndrome
- PMID: 36130736
- PMCID: PMC10092513
- DOI: 10.1002/ejp.2040
Pain-autonomic measures reveal nociceptive sensitization in complex regional pain syndrome
Abstract
Background: Allodynia and hyperalgesia are common signs in individuals with complex regional pain syndrome (CRPS), mainly attributed to sensitization of the nociceptive system. Appropriate diagnostic tools for the objective assessment of such hypersensitivities are still lacking, which are essential for the development of mechanism-based treatment strategies.
Objectives: This study investigated the use of pain-autonomic readouts to objectively detect sensitization processes in CRPS.
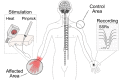
Methods: Twenty individuals with chronic CRPS were recruited for the study alongside 16 age- and sex-matched healthy controls (HC). All individuals underwent quantitative sensory testing and neurophysiological assessments. Sympathetic skin responses (SSRs) were recorded in response to 15 pinprick and 15 noxious heat stimuli of the affected (CRPS hand/foot) and a control area (contralateral shoulder/hand).
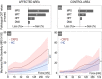
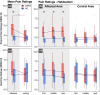
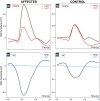
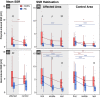
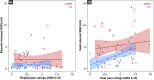
Results: Individuals with CRPS showed increased mechanical pain sensitivity and increased SSR amplitudes compared with HC in response to pinprick and heat stimulation of the affected (p < 0.001), but not in the control area (p > 0.05). Habituation of pinprick-induced SSRs was reduced in CRPS compared to HC in both the affected (p = 0.018) and slightly in the control area (p = 0.048). Habituation of heat-induced SSR was reduced in CRPS in the affected (p = 0.008), but not the control area (p = 0.053).
Conclusions: This is the first study demonstrating clinical evidence that pain-related autonomic responses may represent objective tools to quantify sensitization processes along the nociceptive neuraxis in CRPS (e.g. widespread hyperexcitability). Pain-autonomic readouts could help scrutinize mechanisms underlying the development and maintenance of chronic pain in CRPS and provide valuable metrics to detect mechanism-based treatment responses in clinical trials.
Significance: This study provides clinical evidence that autonomic measures to noxious stimuli can objectively detect sensitization processes along the nociceptive neuraxis in complex regional pain syndrome (CRPS) (e.g. widespread hyperexcitability). Pain-autonomic readouts may represent valuable tools to explore pathophysiological mechanisms in a variety of pain patients and offer novel avenues to help guide mechanism-based therapeutic strategies.
© 2022 The Authors. European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation - EFIC ®.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Priming of the autonomic nervous system after an experimental human pain model.J Neurophysiol. 2023 Aug 1;130(2):436-446. doi: 10.1152/jn.00064.2023. Epub 2023 Jul 5. J Neurophysiol. 2023. PMID: 37405990 Free PMC article.
-
Pain-autonomic interaction: A surrogate marker of central sensitization.Eur J Pain. 2020 Nov;24(10):2015-2026. doi: 10.1002/ejp.1645. Epub 2020 Sep 22. Eur J Pain. 2020. PMID: 32794307
-
Differential endogenous pain modulation in complex-regional pain syndrome.Brain. 2009 Mar;132(Pt 3):788-800. doi: 10.1093/brain/awn346. Epub 2009 Jan 19. Brain. 2009. PMID: 19153154
-
Pain mechanisms in complex regional pain syndrome: a systematic review and meta-analysis of quantitative sensory testing outcomes.J Orthop Surg Res. 2023 Jan 2;18(1):2. doi: 10.1186/s13018-022-03461-2. J Orthop Surg Res. 2023. PMID: 36593515 Free PMC article.
-
[COMPLEX REGIONAL PAIN SYNDROME].Lijec Vjesn. 2015 Sep-Oct;137(9-10):297-306. Lijec Vjesn. 2015. PMID: 26749953 Review. Croatian.
Cited by
-
Nocebo Effect on Pain-Related Autonomic Responses in a State of Experimentally-Induced Sensitization.Eur J Pain. 2025 May;29(5):e70029. doi: 10.1002/ejp.70029. Eur J Pain. 2025. PMID: 40251793 Free PMC article.
-
Understanding inter-individual variability of experimental pain habituation and conditioned pain modulation in healthy individuals.Sci Rep. 2024 Sep 27;14(1):22070. doi: 10.1038/s41598-024-73158-5. Sci Rep. 2024. PMID: 39333624 Free PMC article.
-
Clinical and Molecular Barriers to Understanding the Pathogenesis, Diagnosis, and Treatment of Complex Regional Pain Syndrome (CRPS).Int J Mol Sci. 2025 Mar 11;26(6):2514. doi: 10.3390/ijms26062514. Int J Mol Sci. 2025. PMID: 40141156 Free PMC article.
-
Priming of the autonomic nervous system after an experimental human pain model.J Neurophysiol. 2023 Aug 1;130(2):436-446. doi: 10.1152/jn.00064.2023. Epub 2023 Jul 5. J Neurophysiol. 2023. PMID: 37405990 Free PMC article.
-
Habituation to Pain in Patients with Chronic Pain: Clinical Implications and Future Directions.J Clin Med. 2023 Jun 27;12(13):4305. doi: 10.3390/jcm12134305. J Clin Med. 2023. PMID: 37445339 Free PMC article. Review.
References
-
- Arendt‐Nielsen, L. , Morlion, B. , Perrot, S. , Dahan, A. , Dickenson, A. , Kress, H. G. , Wells, C. , Bouhassira, D. , & Mohr Drewes, A. (2017). Assessment and manifestation of central sensitisation across different chronic pain conditions. European Journal of Pain (United Kingdom), 22, 216–241. - PubMed
-
- Barnes, K. , McNair, N. A. , Harris, J. A. , Sharpe, L. , & Colagiuri, B. (2021). In anticipation of pain: Expectancy modulates corticospinal excitability, autonomic response, and pain perception. Pain, 162, 2287–2296. - PubMed
-
- Baron, R. , Maier, C. , Attal, N. , Binder, A. , Bouhassira, D. , Cruccu, G. , Finnerup, N. B. , Haanpaä, M. , Hansson, P. , Hüllemann, P. , Jensen, T. S. , Freynhagen, R. , Kennedy, J. D. , Magerl, W. , Mainka, T. , Reimer, M. , Rice, A. S. C. , Segerdahl, M. , Serra, J. , … Treede, R. D. (2017). Peripheral neuropathic pain: A mechanism‐related organizing principle based on sensory profiles. Pain, 158, 261–272. - PMC - PubMed
-
- Benarroch, E. E. (2001). Pain‐autonomic interactions: A selective review. Clinical Autonomic Research, 11, 343–349. - PubMed

