Preclinical and randomized clinical evaluation of the p38α kinase inhibitor neflamapimod for basal forebrain cholinergic degeneration
- PMID: 36130946
- PMCID: PMC9492778
- DOI: 10.1038/s41467-022-32944-3
Preclinical and randomized clinical evaluation of the p38α kinase inhibitor neflamapimod for basal forebrain cholinergic degeneration
Abstract
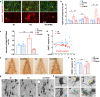
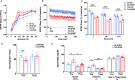
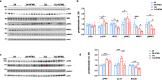
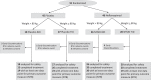
The endosome-associated GTPase Rab5 is a central player in the molecular mechanisms leading to degeneration of basal forebrain cholinergic neurons (BFCN), a long-standing target for drug development. As p38α is a Rab5 activator, we hypothesized that inhibition of this kinase holds potential as an approach to treat diseases associated with BFCN loss. Herein, we report that neflamapimod (oral small molecule p38α inhibitor) reduces Rab5 activity, reverses endosomal pathology, and restores the numbers and morphology of BFCNs in a mouse model that develops BFCN degeneration. We also report on the results of an exploratory (hypothesis-generating) phase 2a randomized double-blind 16-week placebo-controlled clinical trial (Clinical trial registration: NCT04001517/EudraCT #2019-001566-15) of neflamapimod in mild-to-moderate dementia with Lewy bodies (DLB), a disease in which BFCN degeneration is an important driver of disease expression. A total of 91 participants, all receiving background cholinesterase inhibitor therapy, were randomized 1:1 between neflamapimod 40 mg or matching placebo capsules (taken orally twice-daily if weight <80 kg or thrice-daily if weight >80 kg). Neflamapimod does not show an effect in the clinical study on the primary endpoint, a cognitive-test battery. On two secondary endpoints, a measure of functional mobility and a dementia rating-scale, improvements were seen that are consistent with an effect on BFCN function. Neflamapimod treatment is well-tolerated with no study drug associated treatment discontinuations. The combined preclinical and clinical observations inform on the validity of the Rab5-based pathogenic model of cholinergic degeneration and provide a foundation for confirmatory (hypothesis-testing) clinical evaluation of neflamapimod in DLB.
© 2022. The Author(s).
Conflict of interest statement
J.J.A, A.G., and K.B. are employees of EIP Pharma, the sponsor of the clinical study. J.J.A. is also founder of and has stock ownership in EIP Pharma. U.A.G. receives compensation as a scientific consultant to EIP Pharma. S.N.G. has served on Advisory Boards of Jannsen, Acadia, and Sanofi, has received consulting fees from EIP Pharma, and has received funding from the NIH, the DOD CDMRP, the Michael J. Fox Foundation, the FFFPRI, and the Lewy Body Dementia Association. N.D.P. is CEO and co-owner of Brain Research Center. P.M. is a full-time employee at Cogstate Ltd. J.E.H. reports receipt of personal fees in the past 2 years from Actinogen, AlzeCure, Aptinyx, Astra Zeneca, Athira Therapeutics, Axon Neuroscience, Axovant, Bial Biotech, Biogen Idec, BlackThornRx, Boehringer Ingelheim, Brands2life, Cerecin, Cognito, Cognition Therapeutics, Compass Pathways, Corlieve, Curasen, EIP Pharma, Eisai, G4X Discovery, GfHEU, Heptares, Ki Elements, Lundbeck, Lysosome Therapeutics, MyCognition, Neurocentria, Neurocog, Neurodyn Inc, Neurotrack, the NHS, Novartis, Novo Nordisk, Nutricia, Probiodrug, Prothena, Recognify, Regeneron, reMYND, Rodin Therapeutics, Samumed, Sanofi, Signant, Syndesi Therapeutics, Takeda, Vivoryon Therapeutics and Winterlight Labs. In addition, he holds stock options in Neurotrack Inc. and is a joint holder of patents with My Cognition Ltd. C.E.T. has a collaboration contracts with ADx Neurosciences, Quanterix and Eli Lilly; performed contract research or received grants from AC-Immune, Axon Neurosciences, Bioconnect, Bioorchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Grifols, Novo Nordisk, PeopleBio, Roche, Toyama, and Vivoryon; and has had speaker contracts for Roche, Grifols, and Novo Nordisk. P.S. has received consultancy fees (paid to the institution) from AC Immune, Brainstorm Cell, EIP Pharma, ImmunoBrain Checkpoint, Genentech, Novartis, Novo Nordisk. P.S. is also principal investigator of studies with AC Immune, FUJI-film/Toyama, UCB, and Vivoryon; and is an employee of EQT Life Sciences (formerly LSP). The remaining authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

