ZIKV-envelope proteins induce specific humoral and cellular immunity in distinct mice strains
- PMID: 36131132
- PMCID: PMC9492693
- DOI: 10.1038/s41598-022-20183-x
ZIKV-envelope proteins induce specific humoral and cellular immunity in distinct mice strains
Abstract
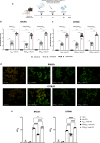
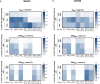
Recent outbreaks of Zika virus (ZIKV) infection have highlighted the need for a better understanding of ZIKV-specific immune responses. The ZIKV envelope glycoprotein (EZIKV) is the most abundant protein on the virus surface and it is the main target of the protective immune response. EZIKV protein contains the central domain (EDI), a dimerization domain containing the fusion peptide (EDII), and a domain that binds to the cell surface receptor (EDIII). In this study, we performed a systematic comparison of the specific immune response induced by different EZIKV recombinant proteins (EZIKV, EDI/IIZIKV or EDIIIZIKV) in two mice strains. Immunization induced high titers of E-specific antibodies which recognized ZIKV-infected cells and neutralized the virus. Furthermore, immunization with EZIKV, EDI/IIZIKV and EDIIIZIKV proteins induced specific IFNγ-producing cells and polyfunctional CD4+ and CD8+ T cells. Finally, we identified 4 peptides present in the envelope protein (E1-20, E51-70, E351-370 and E361-380), capable of inducing a cellular immune response to the H-2Kd and H-2Kb haplotypes. In summary, our work provides a detailed assessment of the immune responses induced after immunization with different regions of the ZIKV envelope protein.
© 2022. The Author(s).
Figures




References
-
- WHO. ZIKA virus, microcephaly, Guillain–Barré syndrome. in (ed. Report, S.) (World Health Organization, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

