Photoresponsive porous materials
- PMID: 36131866
- PMCID: PMC9417539
- DOI: 10.1039/d0na00647e
Photoresponsive porous materials
Abstract
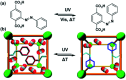
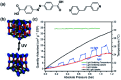
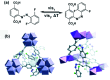
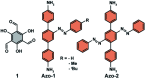
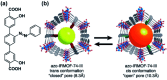
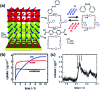
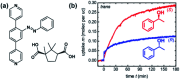
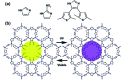
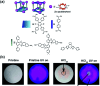
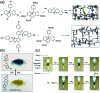
Molecular machines, switches, and motors enable control over nanoscale molecular motion with unprecedented precision in artificial systems. Integration of these compounds into robust material scaffolds, in particular nanostructured solids, is a fabrication strategy for smart materials with unique properties that can be controlled with external stimuli. Here, we describe a subclass of these structures, namely light-responsive porous materials metal-organic frameworks (MOFs), covalent-organic frameworks (COFs), and porous aromatic frameworks (PAFs) appended with molecular photoswitches. In this review, we provide an overview of a broad range of light-responsive porous materials focusing on potential applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
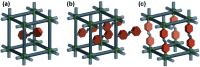
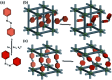
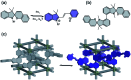


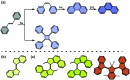
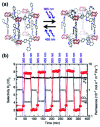
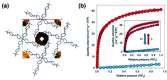
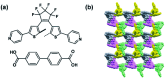

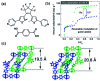

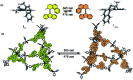
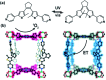







References
-
- Schliwa M., Molecular Motors, Wiley-VCH, Weinheim, 2003
Publication types
LinkOut - more resources
Full Text Sources

