Neurite guidance and neuro-caging on steps and grooves in 2.5 dimensions
- PMID: 36132017
- PMCID: PMC9417336
- DOI: 10.1039/d0na00549e
Neurite guidance and neuro-caging on steps and grooves in 2.5 dimensions
Abstract
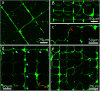
Directed guidance of neurites is a pre-requisite for tailor-made designs of interfaces between cells and semiconducting components. Grayscale lithography, reactive ion etching, and ultraviolet nanoimprint lithography are potent semiconductor industry-compatible techniques for a cost- and time-effective fabrication of modulated surfaces. In this work, neurite outgrowth of murine cerebellar neurons on 2.5D pathways produced with these methods is studied. Structures of micron-sized steps and grooves serve as cell culture platforms. The effects of contact guidance through topography and chemical guidance through selective poly-d-lysine coating on these platforms are analyzed. As a consequence, the herein presented fabrication approach can be utilized to cultivate and to study low-density neuronal networks in 2.5D configuration with a high degree of order.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

