Behavioral analysis of simultaneous photo-electro-catalytic degradation of antibiotic resistant E. coli and antibiotic via ZnO/CuI: a kinetic and mechanistic study
- PMID: 36132120
- PMCID: PMC9419880
- DOI: 10.1039/c9na00483a
Behavioral analysis of simultaneous photo-electro-catalytic degradation of antibiotic resistant E. coli and antibiotic via ZnO/CuI: a kinetic and mechanistic study
Abstract
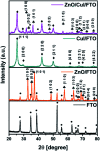
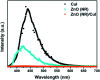
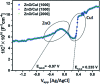
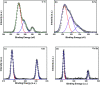
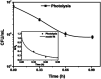
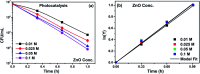
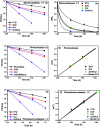
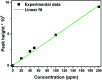
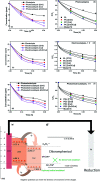
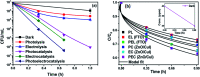
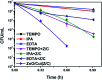
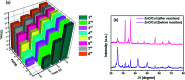
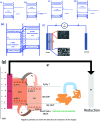
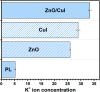
Visible light responsive semiconductor-based photocatalysis is known to be an efficient method for the disinfection of bacterial cells. Here, we address the issue of aqueous contamination by persistent pollutants such as antibiotics and antibiotic resistant bacteria (ARB) from an innovative angle. Simultaneous degradation of an antibiotic (chloramphenicol) and antibiotic resistant bacteria (chloramphenicol resistant E. coli) is performed to observe the effect of the presence of antibiotic in the reaction system when it is required for survival of the bacteria. A p-n junction-based ZnO/CuI composite is shown to demonstrate drastic enhancement in photocatalytic activity due to the inbuilt potential barrier suppressing charge carrier recombination. Moreover, an additional driving force for the suppression of recombination was provided by using a potential bias. Hydrothermally grown ZnO/CuI electrode films were characterized to assess optical, electrochemical, physicochemical and structural properties of the composite. Electrochemical impedance spectroscopy and diffuse reflectance spectroscopy were performed to obtain insights into the band bending, band edge potential, band gap and transmittance of the semiconductors. X-ray-based spectroscopic methods and zeta potential measurement demonstrated the surface properties and surface charges of the moieties in the reaction system, allowing us to deduce justifiable conclusions. A model based on the interaction of photogenerated radicals with the bacteria was developed and rate expressions were used to obtain the rate constants for the experimental results. Photoelectrocatalysis and photocatalysis followed first order rate kinetics; however, due to the unavailability of direct hole attack in photolysis, the electrolysis and electrocatalysis followed Langmuir-Hinshelwood kinetics. Bacterial disinfection was confirmed by K+ ion leaching and by structural changes in the membrane observed by FTIR of the cells after the reaction. We also addressed the issue of bacterial adhesion on the films restricting the mobility of radicals to interact with the bacteria, affecting the reusability of the catalyst films. The present work opens a wide avenue to discuss and address the improvement of the reusability of nanomaterial films for bacterial applications by controlling bacterial adhesion.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Edition F. WHO Chron. 2011;38:104–108.
-
- Ambrosetti B. Campanella L. Palmisano R. J. Environ. Sci. Eng. A. 2015;4:273–281.
LinkOut - more resources
Full Text Sources
Miscellaneous

