What can we learn from more than 1,000 Brazilian patients at risk of hereditary cancer?
- PMID: 36132150
- PMCID: PMC9484549
- DOI: 10.3389/fonc.2022.963910
What can we learn from more than 1,000 Brazilian patients at risk of hereditary cancer?
Abstract
Background: Identifying individuals at a higher risk of developing cancer is a major concern for healthcare providers. Cancer predisposition syndromes are the underlying cause of cancer aggregation and young-onset tumors in many families. Germline genetic testing is underused due to lack of access, but Brazilian germline data associated with cancer predisposition syndromes are needed.
Methods: Medical records of patients referred for genetic counseling at the Oncogenetics Department at the Hospital Sírio-Libanês (Brasília, DF, Brazil) from July 2017 to January 2021 were reviewed. The clinical features and germline findings were described. Detection rates of germline pathogenic/likely pathogenic variant (P/LPV) carriers were compared between international and Brazilian guidelines for genetic testing.
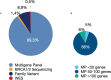
Results: A total of 1,091 individuals from 985 families were included in this study. Most patients (93.5%) had a family history of cancer, including 64% with a family member under 50 with cancer. Sixty-six percent of patients (720/1091) had a personal history of cancer. Young-onset cancers (<50 years old) represented 62% of the patients affected by cancer and 17% had multiple primary cancers. The cohort included patients with 30 different cancer types. Breast cancer was the most prevalent type of cancer (52.6%). Germline testing included multigene panel (89.3%) and family variant testing (8.9%). Approximately 27% (236/879) of the tested patients harbored germline P/LPVs in cancer susceptibility genes. BRCA2, BRCA1, and TP53 were the most frequently reported genes, corresponding to 18.6%, 14.4%, and 13.5% of the positive results, respectively. Genetic testing criteria from international guidelines were more effective in identifying carriers than the Brazilian National Agency of Supplementary Health (ANS) criteria (92% vs. 72%, p<0.001). Forty-six percent of the cancer-unaffected patients who harbored a germline P/LPV (45/98) would not be eligible for genetic testing according to ANS because they did not have a family variant previously identified in a cancer-affected relative.
Conclusion: The high detection rate of P/LPVs in the present study is possibly related to the genetic testing approach with multigene panels and cohort's characteristics, represented mainly by individuals with a personal or family history of young-onset cancer. Testing asymptomatic individuals with suspicious family history may also have contributed to a higher detection rate. A significant number of carriers would not have been identified using ANS criteria for genetic testing.
Keywords: cancer predisposition; cancer risk assessment; genetic testing access; hereditary cancer; multigene analyses.
Copyright © 2022 Leite, Suzuki, Pereira, Machado, Barroso-Sousa, Correa, Moura, Morbeck, Gumz, Faria, Fernandes and Sandoval.
Conflict of interest statement
Author DAS participates in clinical studies sponsored by the company Lilly, and as a speaker at events for AstraZeneca, MSD, Novartis, Roche, Pfizer, Advisory, GSK, Roche. Author AALP reported research involvement with MSD; received honoraria from Servier, MERK, MSD, Novartis, Lilly, AstraZeneca; reported logistic support for educational meetings from AmGen/MSD/Roche; and had advisory role from Servier, Bayer, MERK. Author RB-S reported receiving speaker bureau fees from Agilant, AstraZeneca, Daichi-Sankyo, Eli Lilly, Pfizer, Novartis, Merck, and Roche; he has also served as a consultant/advisor for AstraZeneca, Daichi-Sankyo, Eli Lilly, Libbs, Roche, Merck and has received support for attending medical conferences from AstraZeneca, Eli Lilly, Daichi-Sankyo, and Merck. Author TSC participates in clinical studies sponsored by the company Lilly, Pfizer, Novartis, BMS; and reported receiving speaker bureau fees from AstraZeneca, Novartis, Pfizer, Novartis, Libs. Author IAPM is employed as an advisory board by BMS, Bayer, Astellas, Janssen, MSD, ROCHE, AstraZeneca, participates in clinical studies sponsored by the company BMS, Astellas, Janssen; and reported receiving speaker bureau fees from Novartis, Janssen, ROCHE, Astellas, MSD, BMS, IPSEN, AMGEM, AstraZeneca. Author GdSF is employed as an advisory board by BMS, MSD, ROCHE, Astellas, Boeringher, Bayer, and reported receiving speaker bureau fees from BMS, MSD, ROCHE. DAS participates in clinical studies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Li FP, Fraumeni JF, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res (1988) 48(18):5358–62. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous