Shell thickness dependent photostability studies of green-emitting "Giant" quantum dots
- PMID: 36132372
- PMCID: PMC9417657
- DOI: 10.1039/d1na00663k
Shell thickness dependent photostability studies of green-emitting "Giant" quantum dots
Abstract
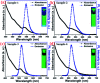
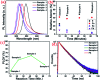
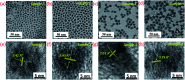
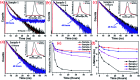
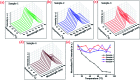
Highly efficient green-emitting core/shell giant quantum dots have been synthesized through a facile "one-pot" gradient alloy approach. Furthermore, an additional ZnS shell was grown using the "Successive Ionic Layer Adsorption and Reaction" (SILAR) method. Due to the faster reactivity of Cd and Se compared to an analogue of Zn and S precursors it is presumed that CdSe nuclei are initially formed as the core and gradient alloy shells simultaneously encapsulate the core in an energy-gradient manner and eventually thick ZnS shells were formed. Using this gradient alloy approach, we have synthesized four different sized green-emitting giant core-shell quantum dots to study their shell thickness-dependent photostability under continuous UV irradiation, and temperature-dependent PL properties of nanocrystals. There was a minimum effect of the UV light exposure on the photostability beyond a certain thickness of the shell. The QDs with a diameter of ≥8.5 nm show substantial improvement in photostability compared to QDs with a diameter ≤ 7.12 nm when continuously irradiated under strong UV light (8 W cm-2, 365 nm) for 48 h. The effect of temperature on the photoluminescence intensities was studied with respect to the shell thickness. There were no apparent changes in PL intensities observed for the QDs ≥ 8.5 nm, on the contrary, for example, QDs with <8.5 nm in diameter (for ∼7.12 nm) show a decrease in PL intensity at higher temperatures ∼ 90 °C. The synthesized green-emitting gradient alloy QDs with superior optical properties can be used for highly efficient green-emitters and are potentially applicable for the fabrication of green LEDs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Highly efficient Blue-Emitting CdSe-derived Core/Shell Gradient Alloy Quantum Dots with Improved Photoluminescent Quantum Yield and Enhanced Photostability.Langmuir. 2017 Apr 18;33(15):3711-3719. doi: 10.1021/acs.langmuir.6b04333. Epub 2017 Apr 6. Langmuir. 2017. PMID: 28363020
-
Study of Shell Thickness-Dependent Charge Transfer Dynamics in Green-Emitting Core/Shell Giant Quantum Dots.Inorg Chem. 2022 Jan 17;61(2):1059-1066. doi: 10.1021/acs.inorgchem.1c03185. Epub 2021 Dec 28. Inorg Chem. 2022. PMID: 34962784
-
Role of shell composition and morphology in achieving single-emitter photostability for green-emitting "giant" quantum dots.J Chem Phys. 2020 Mar 31;152(12):124713. doi: 10.1063/5.0002772. J Chem Phys. 2020. PMID: 32241141
-
Ultrafast dynamics and ultrasensitive single particle spectroscopy of optically robust core/alloy shell semiconductor quantum dots.Phys Chem Chem Phys. 2022 Apr 13;24(15):8578-8590. doi: 10.1039/d1cp05780d. Phys Chem Chem Phys. 2022. PMID: 35355030 Review.
-
Repercussions of the Inner Shell Layer on the Performance of Cd-Free Quantum Dots and Their Light-Emitting Diodes.J Phys Chem Lett. 2024 Jan 11;15(1):201-211. doi: 10.1021/acs.jpclett.3c03137. Epub 2023 Dec 29. J Phys Chem Lett. 2024. PMID: 38157217 Review.
Cited by
-
The Rise and Future of Discrete Organic-Inorganic Hybrid Nanomaterials.ACS Phys Chem Au. 2022 May 28;2(5):364-387. doi: 10.1021/acsphyschemau.2c00018. eCollection 2022 Sep 28. ACS Phys Chem Au. 2022. PMID: 36855686 Free PMC article. Review.
References
-
- Murray C. B. Norris D. J. Bawendi M. G. J. Am. Chem. Soc. 1993;115:8706–8715. doi: 10.1021/ja00072a025. - DOI
-
- Mishra N. Vasavi Dutt V. G. Arciniegas M. P. Chem. Mater. 2019;31:9216–9242. doi: 10.1021/acs.chemmater.8b05363. - DOI
-
- Tong S. W. Mishra N. Su C. L. Nalla V. Wu W. Ji W. Zhang J. Chan Y. Loh K. P. Adv. Funct. Mater. 2014;24:1904–1910. doi: 10.1002/adfm.201303010. - DOI
LinkOut - more resources
Full Text Sources

