Hydrothermally activated TiO2 nanoparticles with a C-dot/g-C3N4 heterostructure for photocatalytic enhancement
- PMID: 36132837
- PMCID: PMC9419518
- DOI: 10.1039/d1na00213a
Hydrothermally activated TiO2 nanoparticles with a C-dot/g-C3N4 heterostructure for photocatalytic enhancement
Abstract
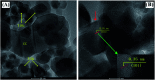
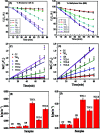
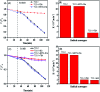
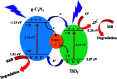
Dye degradation via photocatalysis technology has been investigated intensively to tackle environmental issues and energy crisis concerns. In this study, a newly designed ternary photocatalyst was facilely prepared by a simple one-pot hydrothermal process by directly mixing TiO2 nanoparticles with carbon dots (C-dots) and graphitic carbon nitride (g-C3N4). The optimized precursor treatments and heterostructure components show significantly enhanced photodegradation activity towards organic dyes Rhodamine B (RhB) and methylene blue (MB). Excellent photocatalytic activities were achieved owing to the better attachment of anatase-type TiO2 nanoparticle-aggregations to the C-dots/g-C3N4 (CC) nanocomposite, which impressively displays superhydrophilicity by employing the hydrothermal activation process. FT-IR spectra revealed that the hydrothermal treatment could remarkably increase the coupling interactions between TiO2 nanoparticles and the CC nanosheets within the ternary catalyst, enhancing the photocatalytic activity. Thus, it was concluded that this ternary photocatalyst is highly suitable for the remediation of dye-contaminated wastewater.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Ajmal A. Majeed I. Malik R. N. Idriss H. Nadeem M. A. RSC Adv. 2014;4:37003–37026.
-
- Mahesh K. P. O. Kuo D. H. Appl. Surf. Sci. 2015;357:433–438.
-
- Tan C. Wang W. X. Environ. Pollut. 2017;231:311–318. - PubMed
-
- Bizani E. Fytianos K. Poulios I. Tsiridis V. J. Hazard. Mater. 2006;136:85–94. - PubMed
-
- Lee K. Yoon H. Ahn C. Park J. Y. Jeon S. K. Nanoscale. 2019;11:7025–7040. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

