Physico-chemical properties of selenium-tellurium alloys across the scales
- PMID: 36132844
- PMCID: PMC9416897
- DOI: 10.1039/d1na00087j
Physico-chemical properties of selenium-tellurium alloys across the scales
Abstract
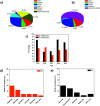
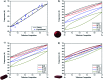
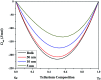
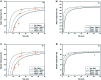
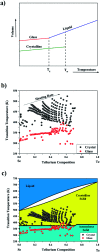
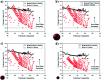
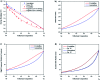
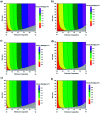
Selenium and tellurium are both energy critical elements as defined by the American Physical Society and the Materials Research Society. When mixed together, both elements form an alloy. The size- and shape-dependent thermal and optical properties of this alloy are investigated in this manuscript by using nano-thermodynamics and machine learning techniques. This alloy is found to have particularly interesting properties for solar cell applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Hurd A. J. et al., Energy-critical elements for sustainable development. MRS Bull. 2012;37:405–410. doi: 10.1557/mrs.2012.54. - DOI
-
- Jaffe R., et al., Energy Critical Elements: Securing Materials for Emerging Technologies, POPA Reports, February 2011, pp. 1–28
-
- Hoffmann J. E. Recovering Selenium and Tellurium from Copper Refinery Slimes. JOM. 1989:33–38. doi: 10.1007/BF03220269. - DOI
LinkOut - more resources
Full Text Sources

