Determining the morphology and concentration of core-shell Au/Ag nanoparticles
- PMID: 36132918
- PMCID: PMC9419198
- DOI: 10.1039/d0na00629g
Determining the morphology and concentration of core-shell Au/Ag nanoparticles
Abstract
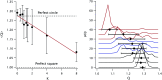
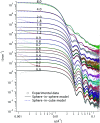
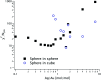
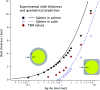
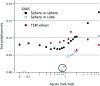
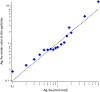
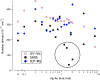
Accurately measuring the shape, structure and concentration of nanoparticles (NPs) is a crucial step towards understanding their formation and a prerequisite for any applications. While determining these parameters for single-metal NPs is by now rather routine, reliably characterizing bimetallic NPs is still a challenge. Using four complementary techniques: transmission electron microscopy (TEM), light absorbance spectroscopy (AS), small-angle X-ray scattering (SAXS) and inductively coupled plasma mass spectrometry (ICP-MS) we study bimetallic nanoparticles obtained by growing a silver shell on top of a gold seed. The initial quasi-spherical objects become faceted and grow into a rounded cube as the molar silver-to-gold ratio K increases. The shape evolution is well described by SAXS and TEM. The shell thickness, overall size polydispersity and number particle concentration obtained by the various methods are in good agreement, validating the use of non-invasive in situ techniques such as AS and SAXS for the study of bimetallic NPs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Park K. Drummy L. F. Vaia R. A. J. Mater. Chem. 2011;21:15608. doi: 10.1039/C1JM12489G. - DOI
-
- Gómez-Graña S. Goris B. Altantzis T. Fernández-López C. Carbó-Argibay E. Guerrero-Martínez A. Almora-Barrios N. López N. Pastoriza-Santos I. Pérez-Juste J. Bals S. Van Tendeloo G. Liz-Marzán L. M. J. Phys. Chem. Lett. 2013;4:2209–2216. doi: 10.1021/jz401269w. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

