Tunable magnetothermal properties of cobalt-doped magnetite-carboxymethylcellulose ferrofluids: smart nanoplatforms for potential magnetic hyperthermia applications in cancer therapy
- PMID: 36133299
- PMCID: PMC9416810
- DOI: 10.1039/d0na00820f
Tunable magnetothermal properties of cobalt-doped magnetite-carboxymethylcellulose ferrofluids: smart nanoplatforms for potential magnetic hyperthermia applications in cancer therapy
Abstract
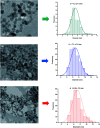
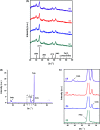
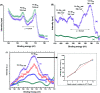
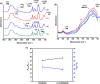
Magnetite nanoparticles are one of the most promising ferrofluids for hyperthermia applications due to the combination of unique physicochemical and magnetic properties. In this study, we designed and produced superparamagnetic ferrofluids composed of magnetite (Fe3O4, MION) and cobalt-doped magnetite (Co x -MION, x = 3, 5, and 10% mol of cobalt) nanoconjugates through an eco-friendly aqueous method using carboxymethylcellulose (CMC) as the biocompatible macromolecular ligand. The effect of the gradual increase of cobalt content in Fe3O4 nanocolloids was investigated in-depth using XRD, XRF, XPS, FTIR, DLS, zeta potential, EMR, and VSM analyses. Additionally, the cytotoxicity of these nanoconjugates and their ability to cause cancer cell death through heat induction were evaluated by MTT assays in vitro. The results demonstrated that the progressive substitution of Co in the magnetite host material significantly affected the magnetic anisotropy properties of the ferrofluids. Therefore, Co-doped ferrite (Co x Fe(3-x)O4) nanoconjugates enhanced the cell-killing activities in magnetic hyperthermia experiments under alternating magnetic field performed with human brain cancer cells (U87). On the other hand, the Co-doping process retained the pristine inverse spinel crystalline structure of MIONs, and it has not significantly altered the average nanoparticle size (ca.∼7.1 ± 1.6 nm). Thus, the incorporation of cobalt into magnetite-polymer nanostructures may constitute a smart strategy for tuning their magnetothermal capability towards cancer therapy by heat generation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interest regarding the publication of this paper.
Figures








References
-
- Ahmed S., Rajak B. L., Gogoi M. and Sarma H. D., in Smart Healthcare for Disease Diagnosis and Prevention, Elsevier Inc., 2020, pp. 153–173
-
- Sathya A. Guardia P. Brescia R. Silvestri N. Pugliese G. Nitti S. Manna L. Pellegrino T. Chem. Mater. 2016;28:1769–1780. doi: 10.1021/acs.chemmater.5b04780. - DOI
LinkOut - more resources
Full Text Sources

