Dual-tracer PET/CT imaging to determine tumor heterogeneity in a patient with metastatic ACTH-secreting neuroendocrine neoplasm: A case report and literature review
- PMID: 36133304
- PMCID: PMC9483167
- DOI: 10.3389/fendo.2022.958442
Dual-tracer PET/CT imaging to determine tumor heterogeneity in a patient with metastatic ACTH-secreting neuroendocrine neoplasm: A case report and literature review
Abstract
Introduction: We present a case of a patient with disseminated ACTH-secreting neuroendocrine neoplasm with biologic heterogeneity between a primary tumor and metastases. The diagnosis was obtained and multidisciplinary management was conducted with a positron emission tomography/computed tomography (PET/CT) scan with Gallium-68 [68Ga]-labeled dodecanetetraacetic acid-tyrosine-3-octreotate ([68Ga]-DOTA-TATE) and Fluor-18 [18F]-fluorodeoxyglucose ([18F]-FDG).
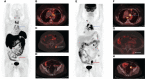
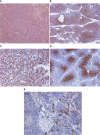
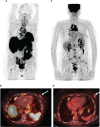
Case report: A PET/CT scan revealed a difference between [68Ga]-DOTA-TATE and [18F]-FDG uptake in primary tumor and several metastases. PET/CT showed high [18F]-FDG uptake and lack of [68Ga]-DOTA-TATE in the primary tumor, whereas both [68Ga]-DOTA-TATE and [18F]-FDG hyperaccumulation were identified in the majority of metastases. Despite positive [68Ga]-DOTA-TATE PET/CT, which is associated with high affinity with the somatostatin receptor 2 subtype, immunohistochemical examination revealed overexpression of the somatostatin receptor 5 subtype only. Perhaps, this explained the ineffectiveness of the treatment with "cold" somatostatin analogs.
Conclusion: This case had an aggressive clinical course, despite cytoreductive surgical treatment and somatostatin analog therapy. PET/CT imaging with two tracers is a molecular tool that demonstrates a biologic heterogeneity between a primary tumor and metastases and yields additional information that may influence the choice of the patient management strategy.
Keywords: PET/CT; [18F]-FDG; [68Ga]-DOTATATE; ectopic Cushing’s syndrome; ovarian NET.
Copyright © 2022 Ryzhkova, Mitrofanova, Tsoy, Grineva and Schlyakhto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer MM declared a past collaboration with the authors DR and LM to the handling editor.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

