Specific gut bacterial and fungal microbiota pattern in the first half of pregnancy is linked to the development of gestational diabetes mellitus in the cohort including obese women
- PMID: 36133313
- PMCID: PMC9484836
- DOI: 10.3389/fendo.2022.970825
Specific gut bacterial and fungal microbiota pattern in the first half of pregnancy is linked to the development of gestational diabetes mellitus in the cohort including obese women
Abstract
Aims: Gestation is linked to changes in gut microbiota composition and function. Since gestational diabetes mellitus (GDM) can develop at any time of the pregnancy, we stratified the women into four groups according to the time and test used for the diagnosis. We focused on the gut microbiota pattern in early pregnancy to detect changes which could be linked to later GDM development.
Methods: We collected stool samples from 104 pregnant women including obese individuals (first trimester body mass index median was 26.73). We divided the women into four groups according to routine screening of fasting plasma glucose (FPG) levels and oral glucose tolerance test (oGTT) in the first and third trimesters, respectively. We processed the stool samples for bacterial 16S rRNA and fungal ITS1 genes sequencing by Illumina MiSeq approach and correlated the gut microbiota composition with plasma short-chain fatty acid levels (SCFA).
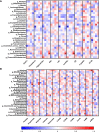
Results: We found that gut bacterial microbiota in the first trimester significantly differs among groups with different GDM onset based on unweighted UniFrac distances (p=0.003). Normoglycemic women had gut microbiota associated with higher abundance of family Prevotellaceae, and order Fusobacteriales, and genus Sutterella. Women diagnosed later during pregnancy either by FGP levels or by oGTT had higher abundances of genera Enterococcus, or Erysipelotrichaceae UCG-003, respectively. We observed significant enrichment of fungal genus Mucor in healthy pregnant women whereas Candida was more abundant in the group of pregnant women with impaired oGTT. Using correlation analysis, we found that Holdemanella negatively correlated with Blautia and Candida abundances and that Escherichia/Shigella abundance positively correlated and Subdoligranulum negatively correlated with plasma lipid levels. Coprococcus, Akkermansia, Methanobrevibacter, Phascolarctobacterium and Alistipes positively correlated with acetate, valerate, 2-hydroxybutyrate and 2-methylbutyrate levels, respectively, in women with GDM.
Conclusions: We conclude that there are significant differences in the gut microbiota composition between pregnant women with and without GDM already at the early stage of pregnancy in our cohort that included also overweight and obese individuals. Specific microbial pattern associated with GDM development during early pregnancy and its correlation to plasma lipid or SCFA levels could help to identify women in higher risk of GDM development.
Keywords: correlation; early diagnosis; microbiome; mycobiome; plasma metabolites; short-chain fatty acids.
Copyright © 2022 Vavreckova, Galanova, Kostovcik, Krystynik, Ivanovova, Roubalova, Jiraskova Zakostelska, Friedecky, Friedecka, Haluzik, Karasek and Kostovcikova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

