Monitoring Cellular Immune Responses after Consumption of Selected Probiotics in Immunocompromised Mice
- PMID: 36133633
- PMCID: PMC9478974
- DOI: 10.5851/kosfa.2022.e44
Monitoring Cellular Immune Responses after Consumption of Selected Probiotics in Immunocompromised Mice
Abstract
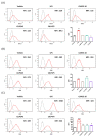
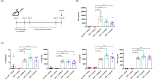
Probiotics are currently considered as one of tools to modulate immune responses under specific clinical conditions. The purpose of this study was to evaluate whether oral administration of three different probiotics (Lactiplantibacillus plantarum CJLP243, CJW55-10, and CJLP475) could evoke a cell-mediated immunity in immunodeficient mice. Before conducting in vivo experiments, we examined the in vitro potency of these probiotics for macrophage activation. After co-culture with these probiotics, bone marrow derived macrophages (BMDMs) produced significant amounts of proinflammatory cytokines including interleukin-6 (IL-6), IL-12, and tumor necrosis factor-α (TNF-α). Levels of inducible nitric oxide synthase (inos) and co-stimulatory molecules (CD80 and CD86) were also upregulated in BMDMs after treatment with some of these probiotics. To establish an immunocompromised animal model, we intraperitoneally injected mice with cyclophosphamide on day 0 and again on day 2. Starting day 3, we orally administered probiotics every day for the last 15 d. After sacrificing experimental mice on day 18, splenocytes were isolated and co-cultured with these probiotics for 3 d to measure levels of several cytokines and immune cell proliferation. Results clearly indicated that the consumption of all three probiotic strains promoted secretion of interferon-γ (IFN-γ), IL-1β, IL-6, IL-12, and TNF-α. NK cell cytotoxicity and proliferation of immune cells were also increased. Taken together, our data strongly suggest that consumption of some probiotics might induce cell-mediated immune responses in immunocompromised mice.
Keywords: animal model; immune response; probiotics.
© Korean Society for Food Science of Animal Resources.
Conflict of interest statement
Yun HS is currently employed by the CJ CheilJedang Corporation, Korea. None of the other authors had any conflict of interests.
Figures


References
-
- Bui VT, Tseng HC, Kozlowska A, Maung PO, Kaur K, Topchyan P, Jewett A. Augmented IFN-γ and TNF-α induced by probiotic bacteria in NK cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol. 2015;6:576. doi: 10.3389/fimmu.2015.00576. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

