Nanonewton forces between Staphylococcus aureus surface protein IsdB and vitronectin
- PMID: 36133863
- PMCID: PMC9419033
- DOI: 10.1039/d0na00636j
Nanonewton forces between Staphylococcus aureus surface protein IsdB and vitronectin
Abstract
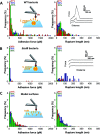
Single-molecule experiments have recently revealed that the interaction between staphylococcal surface proteins and their ligands can be extremely strong, equivalent to the strength of covalent bonds. Here, we report on the unusually high binding strength between Staphylococcus aureus iron-regulated surface determinant B (IsdB) and vitronectin (Vn), an essential human blood protein known to interact with bacterial pathogens. The IsdB-Vn interaction is dramatically strengthened by mechanical tension, with forces up to 2000 pN at a loading rate of 105 pN s-1. In line with this, flow experiments show that IsdB-mediated bacterial adhesion to Vn is enhanced by fluid shear stress. The stress-dependent binding of IsdB to Vn is likely to play a role in promoting bacterial adhesion to human cells under fluid shear stress conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources

