Transparent thermal insulation silica aerogels
- PMID: 36133881
- PMCID: PMC9417477
- DOI: 10.1039/d0na00655f
Transparent thermal insulation silica aerogels
Abstract
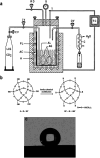
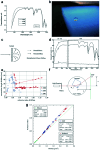
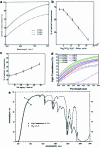
Silica aerogels have received much attention due to their unique nanoporous networks, which consist of nanoscale connective silica particles and high-volume nanoscale pores. This lightweight superinsulation solid materials are synthesized by a 'sol-gel' process involving precursor preparation, gelation, aging and drying. By controlling their synthesis and processing, silica aerogels demonstrate good thermal and acoustic insulation, mechanical strength and optical transparency. In recent years, incorporating transparent and thermal insulation silica aerogels in energy-saving windows is of great interest for both scientific and technological applications. This review introduces the basic principles of thermal and optical properties of silica aerogels and highlights their tunability via synthetic and processing control. In addition, the use of silica aerogels in transparent thermal insulation windows is discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Riffat S. B. Qiu G. Int. J. Low-Carbon Technol. 2013;8:1–6. doi: 10.1093/ijlct/cts001. - DOI
-
- Aegerter M. A., Leventis N. and Koebel M. M., Aerogels handbook, Springer Science & Business Media, 2011
-
- Tabata M. Adachi I. Ishii Y. Kawai H. Sumiyoshi T. Yokogawa H. Nucl. Instrum. Methods Phys. Res., Sect. A. 2010;623:339–341. doi: 10.1016/j.nima.2010.02.241. - DOI
Publication types
LinkOut - more resources
Full Text Sources

