Regioselective thiocyanation of corroles and the synthesis of gold nanoparticle-corrole assemblies
- PMID: 36134003
- PMCID: PMC9419656
- DOI: 10.1039/c9na00671k
Regioselective thiocyanation of corroles and the synthesis of gold nanoparticle-corrole assemblies
Abstract
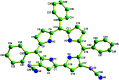
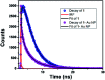
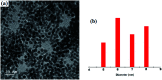
Herein we demonstrate a synthetic protocol for the regioselective thiocyanation of corroles. To the best of our knowledge, thiocyanato appended corrole has never been reported earlier. The resulting thiocyanato appended corrole turned out to be a good corrole based precursor for the facile synthesis of thiol protected gold nanoparticles (Au NPs). The ligand system acts as a good bidentate framework and passivates the gold surface. A strong electronic interaction between the corrole and the gold nanoparticles is manifested by their unique photo physical properties and it also confirms that the binding through β-substitutions has a more pronounced effect even though the corrole rings are face-off to the gold surface.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
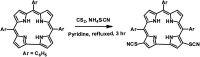





Similar articles
-
The structural chemistry of metallocorroles: combined X-ray crystallography and quantum chemistry studies afford unique insights.Acc Chem Res. 2012 Aug 21;45(8):1203-14. doi: 10.1021/ar200292d. Epub 2012 Mar 23. Acc Chem Res. 2012. PMID: 22444488
-
Photocatalytic C-H Thiocyanation of Corroles: Development of Near-Infrared (NIR)-Emissive Dyes.J Org Chem. 2021 Feb 19;86(4):3324-3333. doi: 10.1021/acs.joc.0c02683. Epub 2021 Feb 1. J Org Chem. 2021. PMID: 33522801
-
Electronic Structure of Corrole Derivatives: Insights from Molecular Structures, Spectroscopy, Electrochemistry, and Quantum Chemical Calculations.Chem Rev. 2017 Feb 22;117(4):3798-3881. doi: 10.1021/acs.chemrev.6b00590. Epub 2017 Feb 13. Chem Rev. 2017. PMID: 28191934
-
Corroles at work: a small macrocycle for great applications.Chem Soc Rev. 2022 Feb 21;51(4):1277-1335. doi: 10.1039/d1cs00662b. Chem Soc Rev. 2022. PMID: 35037929 Review.
-
Development of the Peripheral Functionalization Chemistry of meso-Free Corroles.Chemistry. 2021 Nov 11;27(63):15605-15615. doi: 10.1002/chem.202102267. Epub 2021 Sep 23. Chemistry. 2021. PMID: 34363279 Review.
Cited by
-
Corroles and Hexaphyrins: Synthesis and Application in Cancer Photodynamic Therapy.Molecules. 2020 Jul 29;25(15):3450. doi: 10.3390/molecules25153450. Molecules. 2020. PMID: 32751215 Free PMC article. Review.
-
Assessment of the performance of six indices in predicating the aromaticity of planar porphyrinoids.J Mol Model. 2023 Mar 2;29(3):83. doi: 10.1007/s00894-023-05485-9. J Mol Model. 2023. PMID: 36862263
-
Synthesis, Characterization, and the N Atom Transfer Reactivity of a Nitridochromium(V) Complex Stabilized by a Corrolato Ligand.ACS Omega. 2022 Aug 3;7(32):28138-28147. doi: 10.1021/acsomega.2c02267. eCollection 2022 Aug 16. ACS Omega. 2022. PMID: 35990448 Free PMC article.
References
-
- Giljohann D. A. Seferos D. S. Daniel W. L. Massich M. D. Patel P. C. Mirkin C. A. Angew. Chem., Int. Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. - DOI - PMC - PubMed
- Dreaden E. C. Alkilany A. M. Huang X. Murphy C. J. El-Sayed M. A. Chem. Soc. Rev. 2012;41:2740–2779. doi: 10.1039/C1CS15237H. - DOI - PMC - PubMed
- Li J. J. Zou L. Hartono D. Ong C. N. Bay B. H. Lanry Yung L. Y. Adv. Mater. 2008;20:138–142. doi: 10.1002/adma.200701853. - DOI
-
- Lu Y.-C. Xu Z. Gasteiger H. A. Chen S. Hamad-Schifferli K. Shao-Horn Y. J. Am. Chem. Soc. 2010;132:12170–12171. doi: 10.1021/ja1036572. - DOI - PubMed
- Muszynski R. Seger B. Kamat P. V. J. Phys. Chem. C. 2008;112:5263–5266. doi: 10.1021/jp800977b. - DOI
- Luo J. Wang L. Mott D. Njoki P. N. Lin Y. He T. Xu Z. Wanjana B. N. Lim I. I. S. Zhong C. J. Adv. Mater. 2008;20:4342–4347. doi: 10.1002/adma.200703009. - DOI
-
- Talley C. E. Jackson J. B. Oubre C. Grady N. K. Hollars C. W. Lane S. M. Huser T. R. Nordlander P. Halas N. J. Nano Lett. 2005;5:1569–1574. doi: 10.1021/nl050928v. - DOI - PubMed
- Wustholz K. L. Henry A.-I. McMahon J. M. Freeman R. G. Valley N. Piotti M. E. Natan M. J. Schatz G. C. Van Duyne R. P. J. Am. Chem. Soc. 2010;132:10903–10910. doi: 10.1021/ja104174m. - DOI - PubMed
- Félidj N. Aubard J. Lévi G. Krenn J. R. Hohenau A. Schider G. Leitner A. Aussenegg F. R. Appl. Phys. Lett. 2003;82:3095–3097. doi: 10.1063/1.1571979. - DOI
LinkOut - more resources
Full Text Sources

