Interaction between glyphosate pesticide and amphiphilic peptides for colorimetric analysis
- PMID: 36134354
- PMCID: PMC9400510
- DOI: 10.1039/d2na00345g
Interaction between glyphosate pesticide and amphiphilic peptides for colorimetric analysis
Abstract
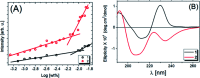
The large-scale use of glyphosate pesticides in food production has attracted attention due to environmental damage and toxicity risks. Several regulatory authorities have established safe limits or concentrations of these pesticides in water and various food products consumed daily. The irreversible inhibition of acetylcholinesterase (AChE) activity is one of the strategies used for pesticide detection. Herein, we found that lipopeptide sequences can act as biomimetic microenvironments of AChE, showing higher catalytic activities than natural enzymes in an aqueous solution, based on IC50 values. These biomolecules contain in the hydrophilic part the amino acids l-proline (P), l-arginine (R), l-tryptophan (W), and l-glycine (G), covalently linked to a hydrophobic part formed by one or two long aliphatic chains. The obtained materials are referred to as compounds 1 and 2, respectively. According to fluorescence assays, 2 is more hydrophobic than 1. The circular dichroism (CD) data present a significant difference in the molar ellipticity values, likely related to distinct conformations assumed by the proline residue in the lipopeptide supramolecular structure in solution. The morphological aspect was further characterized using small-angle X-ray scattering (SAXS) and cryogenic transmission electron microscopy (cryo-TEM), which showed that compounds 1 and 2 self-assembly into cylindrical and planar core-shell structures, respectively. The mimetic AchE behaviour of lipopeptides was confirmed by Ellman's hydrolysis reaction, where the proline residue in the peptides act as a nucleophilic scavenger of organophosphate pesticides. Moreover, the isothermal titration calorimetry (ITC) experiments revealed that host-guest interactions in both systems were dominated by enthalpically-driven thermodynamics. UV-vis kinetic experiments were performed to assess the inhibition of the lipopeptide catalytic activity and the IC50 values were obtained, and we found that the detection limit correlated with the increase in hydrophobicity of the lipopeptides, implying the micellization process is more favorable.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
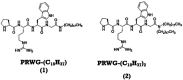




References
-
- Wong A. Silva T. A. Caetano F. R. Bergamini M. F. Marcolino L. H. Fatibello O. Janegitz B. C. C. 2017;3:1–18.
LinkOut - more resources
Full Text Sources

