Targeting cell surface glycans with lectin-coated fluorescent nanodiamonds
- PMID: 36134370
- PMCID: PMC9418452
- DOI: 10.1039/d2na00036a
Targeting cell surface glycans with lectin-coated fluorescent nanodiamonds
Abstract
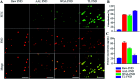
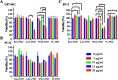
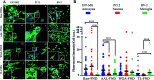
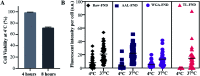
Glycosylation is arguably the most important functional post-translational modification in brain cells and abnormal cell surface glycan expression has been associated with neurological diseases and brain cancers. In this study we developed a novel method for uptake of fluorescent nanodiamonds (FND), carbon-based nanoparticles with low toxicity and easily modifiable surfaces, into brain cell subtypes by targeting their glycan receptors with carbohydrate-binding lectins. Lectins facilitated uptake of 120 nm FND with nitrogen-vacancy centers in three types of brain cells - U87-MG astrocytes, PC12 neurons and BV-2 microglia cells. The nanodiamond/lectin complexes used in this study target glycans that have been described to be altered in brain diseases including sialic acid glycans via wheat (Triticum aestivum) germ agglutinin (WGA), high mannose glycans via tomato (Lycopersicon esculentum) lectin (TL) and core fucosylated glycans via Aleuria aurantia lectin (AAL). The lectin conjugated nanodiamonds were taken up differently by the various brain cell types with fucose binding AAL/FNDs taken up preferentially by glioblastoma phenotype astrocyte cells (U87-MG), sialic acid binding WGA/FNDs by neuronal phenotype cells (PC12) and high mannose binding TL/FNDs by microglial cells (BV-2). With increasing recognition of glycans having a role in many diseases, the lectin bioconjugated nanodiamonds developed here are well suited for further investigation into theranostic applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

