Optimized Decellularization Protocol for Large Peripheral Nerve Segments: Towards Personalized Nerve Bioengineering
- PMID: 36134958
- PMCID: PMC9495622
- DOI: 10.3390/bioengineering9090412
Optimized Decellularization Protocol for Large Peripheral Nerve Segments: Towards Personalized Nerve Bioengineering
Abstract
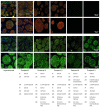
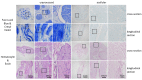
Nerve injuries remain clinically challenging, and allografts showed great promise. Decellularized nerve allografts possess excellent biocompatibility and biological activity. However, the vast majority of decellularization protocols were established for small-size rodent nerves and are not suitable for clinical application. We aimed at developing a new method of decellularizing large-diameter nerves suitable for human transplantation. Repeated rounds of optimization to remove immunogenic material and preserve the extracellular structure were applied to the porcine sciatic nerve. Following optimization, extensive in vitro analysis of the acellular grafts via immunocytochemistry, immunohistology, proteomics and cell transplantation studies were performed. Large segments (up to 8 cm) of the porcine sciatic nerve were efficiently decellularized and histology, microscopy and proteomics analysis showed sufficient preservation of the extracellular matrix, with simultaneous consistent removal of immunogenic material such as myelin, DNA and axons, and axonal growth inhibitory molecules. Cell studies also demonstrated the suitability of these acellular grafts for 3D cell culture studies and translation to future large animal studies and clinical trials. By using non-human donors for peripheral nerve transplantation, significant drawbacks associated with the gold standard can be eliminated while simultaneously preserving the beneficial features of the extracellular matrix.
Keywords: acellular allograft; decellularization; large-gap repair; neurotmesis; peripheral nerve injuries; porcine sciatic nerve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Pfister B.J., Gordon T., Loverde J.R., Kochar A.S., Mackinnon S.E., Cullen D.K. Biomedical Engineering Strategies for Peripheral Nerve Repair: Surgical Applications, State of the Art, and Future Challenges. Crit. Rev. Biomed. Eng. 2011;39:81–124. doi: 10.1615/CritRevBiomedEng.v39.i2.20. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

