Development of Liver-on-Chip Integrating a Hydroscaffold Mimicking the Liver's Extracellular Matrix
- PMID: 36134989
- PMCID: PMC9495334
- DOI: 10.3390/bioengineering9090443
Development of Liver-on-Chip Integrating a Hydroscaffold Mimicking the Liver's Extracellular Matrix
Abstract
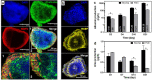
The 3Rs guidelines recommend replacing animal testing with alternative models. One of the solutions proposed is organ-on-chip technology in which liver-on-chip is one of the most promising alternatives for drug screening and toxicological assays. The main challenge is to achieve the relevant in vivo-like functionalities of the liver tissue in an optimized cellular microenvironment. Here, we investigated the development of hepatic cells under dynamic conditions inside a 3D hydroscaffold embedded in a microfluidic device. The hydroscaffold is made of hyaluronic acid and composed of liver extracellular matrix components (galactosamine, collagen I/IV) with RGDS (Arg-Gly-Asp-Ser) sites for cell adhesion. The HepG2/C3A cell line was cultured under a flow rate of 10 µL/min for 21 days. After seeding, the cells formed aggregates and proliferated, forming 3D spheroids. The cell viability, functionality, and spheroid integrity were investigated and compared to static cultures. The results showed a 3D aggregate organization of the cells up to large spheroid formations, high viability and albumin production, and an enhancement of HepG2 cell functionalities. Overall, these results highlighted the role of the liver-on-chip model coupled with a hydroscaffold in the enhancement of cell functions and its potential for engineering a relevant liver model for drug screening and disease study.
Keywords: extracellular matrix; hydroscaffold; liver; organ-on-chip; spheroid.
Conflict of interest statement
HCS Pharma is the BIOMIMESYS® Liver owner and is a partner of ANR MimLiverOnChip (ANR-19-CE19-0020-01). Co-authors Zied Souguir, Victoria Maes, Elodie Vandenhaute, and Nathalie Maubon are employees of HCS Pharma.
Figures






References
-
- Freyer N., Knöspel F., Strahl N., Amini L., Schrade P., Bachmann S., Damm G., Seehofer D., Jacobs F., Monshouwer M., et al. Hepatic differentiation of human induced pluripotent stem cells in a perfused three-dimensional multicompartment bioreactor. Biores. Open Access. 2016;5:235–248. doi: 10.1089/biores.2016.0027. - DOI - PMC - PubMed
-
- Sivaraman A., Leach J.K., Townsend S., Iida T., Hogan B.J., Stolz D.B., Fry R., Samson L.D., Tannenbaum S.R., Griffith L.G. A microscale in vitro physiological model of the liver: Predictive screens for drug metabolism and enzyme induction. Curr. Drug. Metab. 2005;6:569–591. doi: 10.2174/138920005774832632. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

