Hydrogel Beads of Amidoximated Starch and Chitosan as Efficient Sorbents for Inorganic and Organic Compounds
- PMID: 36135261
- PMCID: PMC9498570
- DOI: 10.3390/gels8090549
Hydrogel Beads of Amidoximated Starch and Chitosan as Efficient Sorbents for Inorganic and Organic Compounds
Abstract
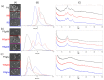
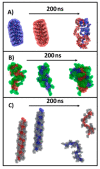
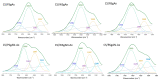
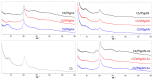
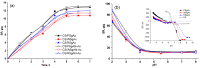
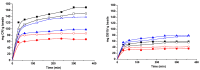
The synthesis of hydrogel beads involving natural polymers is, nowadays, a leading research area. Among natural polymers, starch and chitosan represent two biomolecules with proof of efficiency and low economic impact in various utilization fields. Therefore, herein, the features of hydrogel beads obtained from chitosan and three sorts of starch (potato, wheat and rise starches), grafted with acrylonitrile and then amidoximated, were deeply investigated for their use as sorbents for heavy metal ions and dyes. The hydrogel beads were prepared by ionotropic gelation/covalent cross-linking of chitosan and functionalized starches. The chemical structure of the hydrogel beads was analyzed by FT-IR spectroscopy; their morphology was revealed by optical and scanning electron microscopies, while the influence of the starch functionalization strategies on the crystallinity changes was evaluated by X-ray diffraction. Molecular dynamics simulations were used to reveal the influence of the grafting reactions and grafted structure on the starch conformation in solution and their interactions with chitosan. The sorption capacity of the hydrogel beads was tested in batch experiments, as a function of the beads' features (synthesis protocol, starch sort) and simulated polluted water, which included heavy metal ions (Cu2+, Co2+, Ni2+ and Zn2+) and small organic molecules (Direct Blue 15 and Congo red).
Keywords: covalent cross-linking; grafted starch; ionotropic gelation; molecular dynamics simulation; sorption capacity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wojnárovits L., Földváry C.M., Takács E. Radiation-induced grafting of cellulose for adsoption of hazardous water pollutans: A review. Radiat. Phys. Chem. 2010;79:848–862. doi: 10.1016/j.radphyschem.2010.02.006. - DOI
-
- Chen Q., Yu H., Wang L., ul Abdin Z., Chen Y., Wang J., Zhou W., Yang X., Khan R.U., Zhang H., et al. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015;5:67459–67474. doi: 10.1039/C5RA10849G. - DOI
-
- Loghin D.F., Dragan E.S., Mihai M. Comparative chemical modification of starches as a function of their origin: Synthesis and analysis. Rev. Roum. Chim. 2019;64:915–921. doi: 10.33224/rrch/2019.64.10.11. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

