A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders
- PMID: 36135275
- PMCID: PMC9498590
- DOI: 10.3390/gels8090563
A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders
Abstract
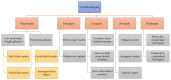
Gels are attractive candidates for drug delivery because they are easily producible while offering sustained and/or controlled drug release through various mechanisms by releasing the therapeutic agent at the site of action or absorption. Gels can be classified based on various characteristics including the nature of solvents used during preparation and the method of cross-linking. The development of novel gel systems for local or systemic drug delivery in a sustained, controlled, and targetable manner has been at the epitome of recent advances in drug delivery systems. Cross-linked gels can be modified by altering their polymer composition and content for pharmaceutical and biomedical applications. These modifications have resulted in the development of stimuli-responsive and functionalized dosage forms that offer many advantages for effective dosing of drugs for Central Nervous System (CNS) conditions. In this review, the literature concerning recent advances in cross-linked gels for drug delivery to the CNS are explored. Injectable and non-injectable formulations intended for the treatment of diseases of the CNS together with the impact of recent advances in cross-linked gels on studies involving CNS drug delivery are discussed.
Keywords: central nervous system; cross-linked gels; injectable cross-linked gels; non-injectable cross-linked gels; spatial drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Polymer based Gels: Recent and Future Applications in Drug Delivery Field.Curr Drug Deliv. 2023;20(9):1288-1313. doi: 10.2174/1567201819666220907124040. Curr Drug Deliv. 2023. PMID: 36082850 Review.
-
Targeted Delivery Strategies of Herbal-Based Nanogels: Advancements and Applications.Curr Drug Targets. 2023;24(16):1260-1270. doi: 10.2174/0113894501275800231103063853. Curr Drug Targets. 2023. PMID: 37953621 Review.
-
Recent advances on small molecular gels: formation mechanism and their application in pharmaceutical fields.Expert Opin Drug Deliv. 2022 Dec;19(12):1597-1617. doi: 10.1080/17425247.2022.2138329. Epub 2022 Nov 23. Expert Opin Drug Deliv. 2022. PMID: 36259939 Review.
-
Promising Polymeric Drug Carriers for Local Delivery: The Case of in situ Gels.Curr Drug Deliv. 2020;17(8):675-693. doi: 10.2174/1567201817666200608145748. Curr Drug Deliv. 2020. PMID: 32510291 Review.
-
Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review.J Control Release. 2021 Jan 10;329:16-35. doi: 10.1016/j.jconrel.2020.11.049. Epub 2020 Nov 28. J Control Release. 2021. PMID: 33259851 Review.
Cited by
-
Recent Insights about the Role of Gels in Organic Photonics and Electronics.Gels. 2023 Nov 4;9(11):875. doi: 10.3390/gels9110875. Gels. 2023. PMID: 37998965 Free PMC article. Review.
-
A review: Carrier-based hydrogels containing bioactive molecules and stem cells for ischemic stroke therapy.Bioact Mater. 2025 Mar 5;49:39-62. doi: 10.1016/j.bioactmat.2025.01.014. eCollection 2025 Jul. Bioact Mater. 2025. PMID: 40124600 Free PMC article. Review.
-
Leveraging flexible pipette-based tool changes to transform liquid handling systems into dual-function sample preparation and imaging platforms.HardwareX. 2025 May 2;22:e00653. doi: 10.1016/j.ohx.2025.e00653. eCollection 2025 Jun. HardwareX. 2025. PMID: 40546787 Free PMC article.
-
New Methacrylated Biopolymer-Based Hydrogels as Localized Drug Delivery Systems in Skin Cancer Therapy.Gels. 2023 May 1;9(5):371. doi: 10.3390/gels9050371. Gels. 2023. PMID: 37232963 Free PMC article.
-
Advances in cellulose-based hydrogels: tunable swelling dynamics and their versatile real-time applications.RSC Adv. 2025 Apr 14;15(15):11688-11729. doi: 10.1039/d5ra00521c. eCollection 2025 Apr 9. RSC Adv. 2025. PMID: 40236573 Free PMC article. Review.
References
-
- O’Loinsigh E., Bose A. Handbook of Behavioral Neuroscience. Volume 29. Elsevier; Amsterdam, The Netherlands: 2019. Regulatory Considerations for the Use of Biomarkers and Personalized Medicine in CNS Drug Development: A European Perspective; pp. 259–275.
-
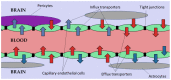
- Li G., Shao K., Umeshappa C.S. Brain Targeted Drug Delivery System. Elsevier; Amsterdam, The Netherlands: 2019. Recent Progress in Blood-Brain Barrier Transportation Research; pp. 33–51.
-
- Vieira A., Filho M., Chaves S.N., Martins W.R., Tolentino G.P., De R., Pereira C., Homem P., De Farias G.L., Fischer B.L., et al. Progressive Resistance Training Improves Bradykinesia, Motor Symptoms and Functional Performance in Patients with Parkinson’s Disease. Clin. Interv. Aging. 2020;15:87–95. doi: 10.2147/CIA.S231359. - DOI - PMC - PubMed
-
- Witika B.A., Poka M.S., Demana P.H., Matafwali S.K., Melamane S., Malungelo Khamanga S.M., Makoni P.A. Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date. Pharmaceutics. 2022;14:836. doi: 10.3390/pharmaceutics14040836. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

