Recombinant Actifensin and Defensin-d2 Induce Critical Changes in the Proteomes of Multidrug-Resistant Pseudomonas aeruginosa and Candida albicans
- PMID: 36135381
- PMCID: PMC9602346
- DOI: 10.1128/spectrum.02062-22
Recombinant Actifensin and Defensin-d2 Induce Critical Changes in the Proteomes of Multidrug-Resistant Pseudomonas aeruginosa and Candida albicans
Abstract
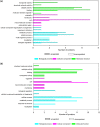
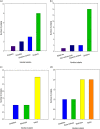
Drug-resistant strains of Pseudomonas aeruginosa and Candida albicans pose serious threats to human health because of their propensity to cause fatal infections. Defensin and defensin-like antimicrobial peptides (AMPs) are being explored as new lines of antimicrobials, due to their broad range of activity, low toxicity, and low pathogen resistance. Defensin-d2 and actifensin are AMPs from spinach and Actinomyces ruminicola, respectively, whose mechanisms of action are yet to be clearly elucidated. This study investigated the mechanisms of action of the recombinant AMPs through label-free quantitative proteomics. The data are available at PRIDE with accession number PXD034169. A total of 28 and 9 differentially expressed proteins (DEPs) were identified in the treated P. aeruginosa and C. albicans, respectively, with a 2-fold change threshold and P values of <0.05. Functional analysis revealed that the DEPs were involved in DNA replication and repair, translation, and membrane transport in P. aeruginosa, while they were related mainly to oxidative phosphorylation, RNA degradation, and energy metabolism in C. albicans. Protein-protein interactions showed that the DEPs formed linear or interdependent complexes with one another, indicative of functional interaction. Subcellular localization indicated that the majority of DEPs were cytoplasmic proteins in P. aeruginosa, while they were of nuclear or mitochondrial origin in C. albicans. These results show that recombinant defensin-d2 and actifensin can elicit complex multiple organism responses that cause cell death in P. aeruginosa and C. albicans. IMPORTANCE AMPs are considered essential alternatives to conventional antimicrobials because of their broad-spectrum efficacy and low potential for resistance by target cells. In this study, we established that the recombinant AMPs defensin-d2 and actifensin exert proteomic changes in P. aeruginosa and C. albicans within 1 h after treatment. We also found that the DEPs in peptide-treated P. aeruginosa are related to ion transport and homeostasis, molecular functions including nucleic and amino acid metabolism, and structural biogenesis and activity, while the DEPs in treated C. albicans are mainly involved in membrane synthesis and mitochondrial metabolism. Our results also highlight ATP synthase as a potential drug target for multidrug-resistant P. aeruginosa and C. albicans.
Keywords: Candida albicans; Pseudomonas aeruginosa; actifensin; antimicrobial mechanism; antimicrobial peptides; proteomics; spinach defensin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Harris AD, Jackson SS, Robinson G, Pineles L, Leekha S, Thom KA, Wang Y, Doll M, Pettigrew MM, Johnson JK. 2016. Pseudomonas aeruginosa colonization in the intensive care unit: prevalence, risk factors, and clinical outcomes. Infect Control Hosp Epidemiol 37:544–548. doi:10.1017/ice.2015.346. - DOI - PMC - PubMed
-
- Shrivastava SR, Shrivastava PS, Ramasamy J. 2018. World Health Organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J Med Soc 32:76–77. doi:10.4103/jms.jms_25_17. - DOI
-
- Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi:10.1093/cid/ciw691. - DOI - PMC - PubMed
-
- Kipanga PN, Liu M, Panda SK, Mai AH, Veryser C, van Puyvelde L, de Borggraeve WM, van Dijck P, Matasyoh J, Luyten W. 2020. Biofilm inhibiting properties of compounds from the leaves of Warburgia ugandensis Sprague subsp ugandensis against Candida and staphylococcal biofilms. J Ethnopharmacol 248:112352. doi:10.1016/j.jep.2019.112352. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

