Evidencing New Roles for the Glycosyl-Transferase Cps1 in the Phytopathogenic Fungus Botrytis cinerea
- PMID: 36135623
- PMCID: PMC9500679
- DOI: 10.3390/jof8090899
Evidencing New Roles for the Glycosyl-Transferase Cps1 in the Phytopathogenic Fungus Botrytis cinerea
Abstract
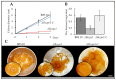
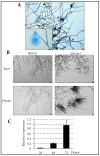
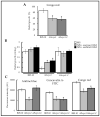
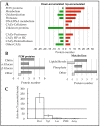
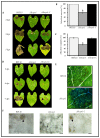
The fungal cell wall occupies a central place in the interaction between fungi and their environment. This study focuses on the role of the putative polysaccharide synthase Cps1 in the physiology, development and virulence of the grey mold-causing agent Botrytis cinerea. Deletion of the Bccps1 gene does not affect the germination of the conidia (asexual spores) or the early mycelial development, but it perturbs hyphal expansion after 24 h, revealing a two-phase hyphal development that has not been reported so far. It causes a severe reduction of mycelial growth in a solid medium and modifies hyphal aggregation into pellets in liquid cultures. It strongly impairs plant penetration, plant colonization and the formation of sclerotia (survival structures). Loss of the BcCps1 protein associates with a decrease in glucans and glycoproteins in the fungus cell wall and the up-accumulation of 132 proteins in the mutant's exoproteome, among which are fungal cell wall enzymes. This is accompanied by an increased fragility of the mutant mycelium, an increased sensitivity to some environmental stresses and a reduced adhesion to plant surface. Taken together, the results support a significant role of Cps1 in the cell wall biology of B. cinerea.
Keywords: Botrytis; adhesion; fungal cell wall; glycosyl-transferase; secretomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

